Modulation of Energy Metabolism Is Important for Low-Oxygen Stress Adaptation in Brassicaceae Species
- PMID: 32150906
- PMCID: PMC7084654
- DOI: 10.3390/ijms21051787
Modulation of Energy Metabolism Is Important for Low-Oxygen Stress Adaptation in Brassicaceae Species
Abstract
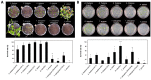
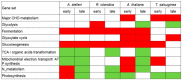
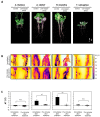
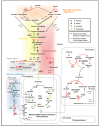
Low-oxygen stress, mainly caused by soil flooding, is a serious abiotic stress affecting crop productivity worldwide. To understand the mechanisms of low-oxygen stress responses and adaptation of plants, we characterized and compared low-oxygen responses in six species with different accessions of the Brassicaceae family. Based on the growth and survival responses to submergence or low-oxygen condition, these accessions could be divided into three groups: (i) Highly tolerant species (Rorippa islandica and Arabis stelleri); (ii) moderately tolerant species (Arabidopsis thaliana [esk-1, Ler, Ws and Col-0 ecotype]); and (iii) intolerant species (Thlaspi arvense, Thellungiella salsuginea [Shandong and Yukon ecotype], and Thellungiella parvula). Gene expression profiling using Operon Arabidopsis microarray was carried out with RNA from roots of A. thaliana (Col-0), A. stelleri, R. islandica, and T. salsuginea (Shandong) treated with low-oxygen stress (0.1% O2/99.9% N2) for 0, 1, 3, 8, 24, and 72 h. We performed a comparative analysis of the gene expression profiles using the gene set enrichment analysis (GSEA) method. Our comparative analysis suggested that under low-oxygen stress each species distinctively reconfigures the energy metabolic pathways including sucrose-starch metabolism, glycolysis, fermentation and nitrogen metabolism, tricarboxylic acid flow, and fatty acid degradation via beta oxidation and glyoxylate cycle. In A. thaliana, a moderately tolerant species, the dynamical reconfiguration of energy metabolisms occurred in the early time points of low-oxygen treatment, but the energy reconfiguration in the late time points was not as dynamic as in the early time points. Highly tolerant A. stelleri appeared to have high photosynthesis capacity that could produce more O2 and in turn additional ATP energy to cope with energy depletion caused by low-oxygen stress. R. islandica seemed to retain some ATP energy produced by anaerobic energy metabolism during a prolonged period of low-oxygen conditions. Intolerant T. salsuginea did not show significant changes in the expression of genes involved in anaerobic energy metabolisms. These results indicate that plants developed different energy metabolisms to cope with the energy crisis caused by low-oxygen stress.
Keywords: Brassicaceae; energy metabolism; gene set enrichment analysis (GSEA); hypoxia; low oxygen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
- 201204010305010010500/Research Cooperating Program for Agricultural Science & Technology Development
- 2017K1A1A2013146/the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (MSIT)
- PJ01330101/Next-Generation BioGreen21 Program by the Rural Development Administration, Korea
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

