2019 FDA TIDES (Peptides and Oligonucleotides) Harvest
- PMID: 32151051
- PMCID: PMC7151716
- DOI: 10.3390/ph13030040
2019 FDA TIDES (Peptides and Oligonucleotides) Harvest
Abstract
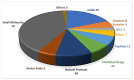
2019 has been an excellent year in terms of peptides and oligonucleotides (TIDES) approved by the FDA. Despite the drop in the number of total drugs approved by the FDA in 2019 in comparison with 2018 (48 vs. 59), the total number of TIDES authorized increased (seven vs. three). Year after year, TIDES are increasingly present in therapy, as imaging agents, theragnostic and constituent moieties of other complex drugs, such as antibody drug conjugates. This means a consolidation of these kinds of drugs in the pharmaceutical arena, paving the way in the coming years for the approval of others for diverse medical indications. Here the TIDES approved in 2019 are analyzed in terms of chemical structure, medical target, mode of action, and adverse effects.
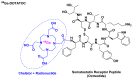
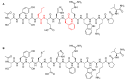
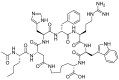
Keywords: 68Ga-DOTATOC; DOTATOC; afamelanotide; bremelanotide; drugs; enfortumab vedotin; givosiran; golodirsen; oligonucleotides; peptides; pharmaceutical market; polatuzumab vedotin.
Conflict of interest statement
References
Figures


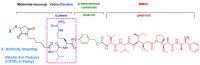
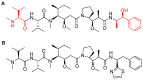
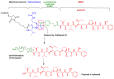
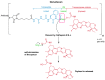
References
-
- New Drug Therapy Approvals 2019. [(accessed on 27 February 2020)];2019 Available online: https://www.fda.gov/media/134493/download.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

