Exposure to Mono-n-Butyl Phthalate in Women with Endometriosis and Its Association with the Biological Effects on Human Granulosa Cells
- PMID: 32151056
- PMCID: PMC7084286
- DOI: 10.3390/ijms21051794
Exposure to Mono-n-Butyl Phthalate in Women with Endometriosis and Its Association with the Biological Effects on Human Granulosa Cells
Abstract
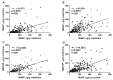
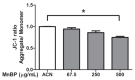
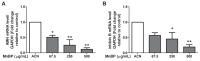
To study the association between urinary phthalate metabolite levels, endometriosis, and their effects on human granulosa cells, we recruited patients who underwent laparoscopy to confirm endometriosis (n = 123) and control patients (n = 78). Liquid chromatography-tandem mass spectrometry was used to measure the following five urinary phthalate metabolites: mono-n-butyl phthalate (MnBP), mono(2-ethylhexyl) phthalate, monobenzyl phthalate, mono(2-ethyl-5-oxo-hexyl) phthalate, and mono(2-ethyl-5-hydroxyhexyl) phthalate. Urinary MnBP levels were higher in patients with endometriosis than in controls after multivariable logistic regression including the number of deliveries, body mass index, and use of medicine as covariables. MnBP correlates with other phthalate metabolites. Previous studies found that endometriosis was a detrimental condition for granulosa cells. In our study, we observed whether MnBP affected granulosa cells. MnBP treatment altered the gene expression of BIRC5, BUB1B, CDC20, cyclin B1, IL-1β, TNF-α, inhibin-B, StAR, and P450ssc and attenuated the ratio of the mitochondrial membrane potential in human granulosa cells. Moreover, MnBP decreased the expression of the anti-Mullerian hormone. These findings suggest that MnBP concentration is associated with endometriosis and may affect the health and steroidogenesis of human granulosa cells.
Keywords: anti-Mullerian hormone; granulosa cells; mitochondrial membrane potential; mono-n-butyl phthalate (MnBP), endometriosis; phthalate.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

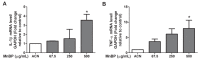
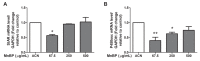
References
-
- Buck Louis G.M., Peterson C.M., Chen Z., Croughan M., Sundaram R., Stanford J., Varner M.W., Kennedy A., Giudice L., Fujimoto V.Y., et al. Bisphenol A and phthalates and endometriosis: The Endometriosis: Natural History, Diagnosis and Outcomes Study. Fertil. Steril. 2013;100:162–169. doi: 10.1016/j.fertnstert.2013.03.026. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

