Kynurenic Acid Protects Against Ischemia/Reperfusion-Induced Retinal Ganglion Cell Death in Mice
- PMID: 32151061
- PMCID: PMC7084183
- DOI: 10.3390/ijms21051795
Kynurenic Acid Protects Against Ischemia/Reperfusion-Induced Retinal Ganglion Cell Death in Mice
Abstract
Background: Glaucoma is an optic neuropathy and involves the progressive degeneration of retinal ganglion cells (RGCs), which leads to blindness in patients. We investigated the role of the neuroprotective kynurenic acid (KYNA) in RGC death against retinal ischemia/reperfusion (I/R) injury.
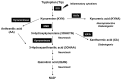
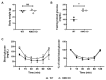
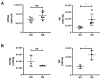
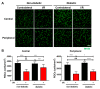
Methods: We injected KYNA intravenously or intravitreally to mice. We generated a knockout mouse strain of kynurenine 3-monooxygenase (KMO), an enzyme in the kynurenine pathway that produces neurotoxic 3-hydroxykynurenine. To test the effect of mild hyperglycemia on RGC protection, we used streptozotocin (STZ) induced diabetic mice. Retinal I/R injury was induced by increasing intraocular pressure for 60 min followed by reperfusion and RGC numbers were counted in the retinal flat mounts.
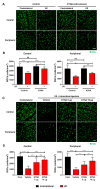
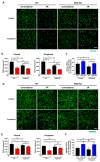
Results: Intravenous or intravitreal administration of KYNA protected RGCs against I/R injury. The I/R injury caused a greater loss of RGCs in wild type than in KMO knockout mice. KMO knockout mice had mildly higher levels of fasting blood glucose than wild type mice. Diabetic mice showed significantly lower loss of RGCs when compared with non-diabetic mice subjected to I/R injury.
Conclusion: Together, our study suggests that the absence of KMO protects RGCs against I/R injury, through mechanisms that likely involve higher levels of KYNA and glucose.
Keywords: diabetes; ischemia/reperfusion; kynurenic acid; kynurenine 3-monooxygenase; neuroprotection; retinal ganglion cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

