MicroRNA-361-Mediated Inhibition of HSP90 Expression and EMT in Cervical Cancer Is Counteracted by Oncogenic lncRNA NEAT1
- PMID: 32151082
- PMCID: PMC7140536
- DOI: 10.3390/cells9030632
MicroRNA-361-Mediated Inhibition of HSP90 Expression and EMT in Cervical Cancer Is Counteracted by Oncogenic lncRNA NEAT1
Abstract
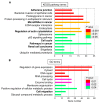
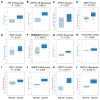
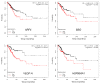
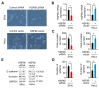
Epithelial-mesenchymal transition (EMT) is a key process contributing to cervical cancer (CC) metastasis, and microRNAs (miRNAs) modulate the expression of genes implicated in EMT. However, the accurate role of miR-361 in CC-associated EMT and the mechanisms underlying its function in CC remains largely unknown. The functional roles of miR-361 in CC cells were explored by a series of cell functional assays. Luciferase reporter assays were used to demonstrate the potential interaction between miR-361, HSP90, and long non-coding RNA (lncRNA) NEAT1. We detected a reduction of miR-361 expression in CC tissues compared with normal tissues, and miR-361 overexpression inhibited invasion and EMT phenotypes of CC cells by directly targeting a key EMT activator HSP90. Additionally, we detected significantly higher levels of HSP90 in CC tissues compared with normal tissues, and high expression of HSP90 predicted a poorer prognosis. We further identified NEAT1 as a significantly upregulated lncRNA in CC tissues and high expression of NEAT1 was associated with worse survival in CC patients. NEAT1 directly repressed miR-361 expression and played an oncogenic role in CC cell invasion and sphere formation. Conclusions: These results demonstrated that miR-361 directly targets HSP90 to inhibit the invasion and EMT features, and NEAT1 functions as an oncogenic lncRNA that suppresses miR-361 expression and induces EMT and sphere formation in CC cells, thus providing critical insights into the molecular pathways operating in this malignancy.
Keywords: EMT; HSP90AA1; NEAT1; cervical cancer; long non-coding RNA; microRNA-361.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures






References
-
- Konno Y., Dong P., Xiong Y., Suzuki F., Lu J., Cai M., Watari H., Mitamura T., Hosaka M., Hanley S.J., et al. MicroRNA-101 targets EZH2, MCL-1 and FOS to suppress proliferation, invasion and stem cell-like phenotype of aggressive endometrial cancer cells. Oncotarget. 2014;5:6049–6062. doi: 10.18632/oncotarget.2157. - DOI - PMC - PubMed

