FAM13A Represses AMPK Activity and Regulates Hepatic Glucose and Lipid Metabolism
- PMID: 32151973
- PMCID: PMC7063182
- DOI: 10.1016/j.isci.2020.100928
FAM13A Represses AMPK Activity and Regulates Hepatic Glucose and Lipid Metabolism
Abstract
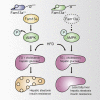
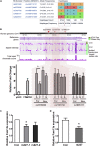
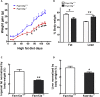
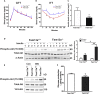
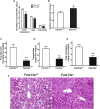
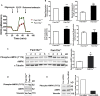
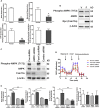
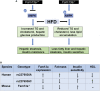
Obesity commonly co-exists with fatty liver disease with increasing health burden worldwide. Family with Sequence Similarity 13, Member A (FAM13A) has been associated with lipid levels and fat mass by genome-wide association studies (GWAS). However, the function of FAM13A in maintaining metabolic homeostasis in vivo remains unclear. Here, we demonstrated that rs2276936 in this locus has allelic-enhancer activity in massively parallel reporter assays (MPRA) and reporter assay. The DNA region containing rs2276936 regulates expression of endogenous FAM13A in HepG2 cells. In vivo, Fam13a-/- mice are protected from high-fat diet (HFD)-induced fatty liver accompanied by increased insulin sensitivity and reduced glucose production in liver. Mechanistically, loss of Fam13a led to the activation of AMP-activated protein kinase (AMPK) and increased mitochondrial respiration in primary hepatocytes. These findings demonstrate that FAM13A mediates obesity-related dysregulation of lipid and glucose homeostasis. Targeting FAM13A might be a promising treatment of obesity and fatty liver disease.
Keywords: Biological Sciences; Cell Biology; Functional Aspects of Cell Biology.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures
References
-
- Castaldi P.J., Guo F., Qiao D., Du F., Naing Z.Z.C., Li Y., Pham B., Mikkelsen T.S., Cho M.H., Silverman E.K. Identification of functional variants in the FAM13A chronic obstructive pulmonary disease genome-wide association study locus by massively parallel reporter assays. Am. J.Respir.Crit. Care Med. 2019;199:52–61. - PMC - PubMed
-
- Cokorinos E.C., Delmore J., Reyes A.R., Albuquerque B., Kjobsted R., Jorgensen N.O., Tran J.L., Jatkar A., Cialdea K., Esquejo R.M. Activation of skeletal muscle AMPK promotes glucose disposal and glucose lowering in non-human primates and mice. Cell Metab. 2017;25:1147–1159.e10. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

