Assessing Risks of Polypharmacy Involving Medications With Anticholinergic Properties
- PMID: 32152019
- PMCID: PMC7062487
- DOI: 10.1370/afm.2501
Assessing Risks of Polypharmacy Involving Medications With Anticholinergic Properties
Abstract
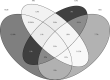
Purpose: Anticholinergic burden (ACB), the cumulative effect of anticholinergic medications, is associated with adverse outcomes in older people but is less studied in middle-aged populations. Numerous scales exist to quantify ACB. The aims of this study were to quantify ACB in a large cohort using the 10 most common anticholinergic scales, to assess the association of each scale with adverse outcomes, and to assess overlap in populations identified by each scale.
Methods: We performed a longitudinal analysis of the UK Biobank community cohort (502,538 participants, baseline age: 37-73 years, median years of follow-up: 6.2). The ACB was calculated at baseline using 10 scales. Baseline data were linked to national mortality register records and hospital episode statistics. The primary outcome was a composite of all-cause mortality and major adverse cardiovascular event (MACE). Secondary outcomes were all-cause mortality, MACE, hospital admission for fall/fracture, and hospital admission with dementia/delirium. Cox proportional hazards models (hazard ratio [HR], 95% CI) quantified associations between ACB scales and outcomes adjusted for age, sex, socioeconomic status, body mass index, smoking status, alcohol use, physical activity, and morbidity count.
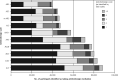
Results: Anticholinergic medication use varied from 8% to 17.6% depending on the scale used. For the primary outcome, ACB was significantly associated with all-cause mortality/MACE for each scale. The Anticholinergic Drug Scale was most strongly associated with mortality/MACE (HR = 1.12; 95% CI, 1.11-1.14 per 1-point increase in score). The ACB was significantly associated with all secondary outcomes. The Anticholinergic Effect on Cognition scale was most strongly associated with dementia/delirium (HR = 1.45; 95% CI, 1.3-1.61 per 1-point increase).
Conclusions: The ACB was associated with adverse outcomes in a middle- to older-aged population. Populations identified and effect size differed between scales. Scale choice influenced the population identified as potentially requiring reduction in ACB in clinical practice or intervention trials.
Keywords: anticholinergic burden; cardiovascular events; mortality; multimorbidity; polypharmacy.
© 2020 Annals of Family Medicine, Inc.
Figures
References
-
- Roe CM, Anderson MJ, Spivack B. Use of anticholinergic medications by older adults with dementia. J Am Geriatr Soc. 2002; 50(5): 836–842. - PubMed
-
- Tune LE. Anticholinergic effects of medication in elderly patients. J Clin Psychiatry. 2001; 62(Suppl 21): 11–14. - PubMed
-
- Boustani M, Campbell N, Munger S, Maidment I, Fox C. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008; 4(3): 311–320.
-
- Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med. 2008; 168(5): 508–513. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
