Age-Dependent Effects of Type I and Type III IFNs in the Pathogenesis of Bordetella pertussis Infection and Disease
- PMID: 32152071
- PMCID: PMC7141952
- DOI: 10.4049/jimmunol.1900912
Age-Dependent Effects of Type I and Type III IFNs in the Pathogenesis of Bordetella pertussis Infection and Disease
Abstract
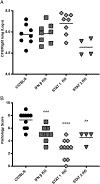
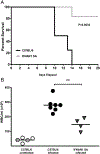
Type I and III IFNs play diverse roles in bacterial infections, being protective for some but deleterious for others. Using RNA-sequencing transcriptomics we investigated lung gene expression responses to Bordetella pertussis infection in adult mice, revealing that type I and III IFN pathways may play an important role in promoting inflammatory responses. In B. pertussis-infected mice, lung type I/III IFN responses correlated with increased proinflammatory cytokine expression and with lung inflammatory pathology. In mutant mice with increased type I IFN receptor (IFNAR) signaling, B. pertussis infection exacerbated lung inflammatory pathology, whereas knockout mice with defects in type I IFN signaling had lower levels of lung inflammation than wild-type mice. Curiously, B. pertussis-infected IFNAR1 knockout mice had wild-type levels of lung inflammatory pathology. However, in response to infection these mice had increased levels of type III IFN expression, neutralization of which reduced lung inflammation. In support of this finding, B. pertussis-infected mice with a knockout mutation in the type III IFN receptor (IFNLR1) and double IFNAR1/IFNLR1 knockout mutant mice had reduced lung inflammatory pathology compared with that in wild-type mice, indicating that type III IFN exacerbates lung inflammation. In marked contrast, infant mice did not upregulate type I or III IFNs in response to B. pertussis infection and were protected from lethal infection by increased type I IFN signaling. These results indicate age-dependent effects of type I/III IFN signaling during B. pertussis infection and suggest that these pathways represent targets for therapeutic intervention in pertussis.
Copyright © 2020 by The American Association of Immunologists, Inc.
Conflict of interest statement
Disclosures
The authors have no financial conflicts of interest.
Figures





References
-
- Clark TA 2014. Changing pertussis epidemiology: everything old is new again. J. Infect. Dis 209: 978–981. - PubMed
-
- Heininger U, Klich K, Stehr K, and Cherry JD. 1997. Clinical findings in Bordetella pertussis infections: results of a prospective multicenter surveillance study. Pediatrics 100: E10. - PubMed
-
- Paddock CD, Sanden GN, Cherry JD, Gal AA, Langston C, Tatti KM, Wu KH, Goldsmith CS, Greer PW, Montague JL, et al. 2008. Pathology and pathogenesis of fatal Bordetella pertussis infection in infants. Clin. Infect. Dis 47: 328–338. - PubMed
-
- Somerville RL, Grant CC, Grimwood K, Murdoch D, Graham D, Jackson P, Meates-Dennis M, Nicholson R, and Purvis D. 2007. Infants hospitalised with pertussis: estimating the true disease burden. J. Paediatr. Child Health 43: 617–622. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

