Effects of Tenofovir on the Single-Dose Pharmacokinetics of Intravenous Morinidazole in Healthy Chinese Subjects
- PMID: 32152080
- PMCID: PMC7179596
- DOI: 10.1128/AAC.02067-19
Effects of Tenofovir on the Single-Dose Pharmacokinetics of Intravenous Morinidazole in Healthy Chinese Subjects
Abstract
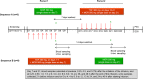
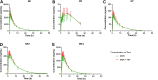
The effects of multiple-dose administration of tenofovir disoproxil fumarate (TDF) on the pharmacokinetics of morinidazole (MOR) were compared in healthy subjects. MOR exposure was similar, with an area under the curve from 0 h to infinity (AUC0-∞) treatment ratio for MOR+TDF/MOR of 1.01 (90% confidence interval, 0.97 to 1.06). No relevant differences were observed regarding plasma exposure of metabolites. Renal clearances of MOR and its metabolites were not affected by TDF. No unexpected safety or tolerability issues were observed.
Keywords: clinical pharmacokinetics; drug-drug interaction; morinidazole; organic anion transporter; tenofovir.
Copyright © 2020 Wu et al.
Figures

Similar articles
-
Assessment of Drug Interaction Potential Between the Hepatitis C Virus Direct-Acting Antiviral Agents Elbasvir/Grazoprevir and the Nucleotide Analog Reverse-Transcriptase Inhibitor Tenofovir Disoproxil Fumarate.Clin Pharmacol Drug Dev. 2019 Oct;8(7):962-970. doi: 10.1002/cpdd.701. Epub 2019 Jun 7. Clin Pharmacol Drug Dev. 2019. PMID: 31173674 Clinical Trial.
-
Effects of renal impairment on the pharmacokinetics of morinidazole: uptake transporter-mediated renal clearance of the conjugated metabolites.Antimicrob Agents Chemother. 2014 Jul;58(7):4153-61. doi: 10.1128/AAC.02414-14. Epub 2014 May 12. Antimicrob Agents Chemother. 2014. PMID: 24820074 Free PMC article.
-
Effects of rifampin and ketoconazole on pharmacokinetics of morinidazole in healthy chinese subjects.Antimicrob Agents Chemother. 2014 Oct;58(10):5987-93. doi: 10.1128/AAC.03382-14. Epub 2014 Jul 28. Antimicrob Agents Chemother. 2014. PMID: 25070100 Free PMC article.
-
Pharmacokinetics of oral tenofovir disoproxil fumarate in pregnancy and lactation: a systematic review.Antivir Ther. 2019;24(7):529-540. doi: 10.3851/IMP3341. Antivir Ther. 2019. PMID: 31868655
-
Development of tenofovir disoproxil fumarate resistance after complete viral suppression in a patient with treatment-naïve chronic hepatitis B: A case report and review of the literature.World J Gastroenterol. 2018 May 7;24(17):1919-1924. doi: 10.3748/wjg.v24.i17.1919. World J Gastroenterol. 2018. PMID: 29740207 Free PMC article. Review.
Cited by
-
Simultaneous determination of five nitroimidazole antimicrobials in pharmaceuticals using HPLC-ESM and QAMS methods.BMC Chem. 2025 Feb 14;19(1):40. doi: 10.1186/s13065-025-01409-1. BMC Chem. 2025. PMID: 39953570 Free PMC article.
References
-
- Lv A. October 2008 Usage of alpha-(morpholin-1-base) methyl-2-methyl-5-azathio-1-alcohol in preparation of anti-trichomoniasis and anti-ameba medicines. China patent ZL 200510134254.3.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

