Effects of Tenofovir on the Single-Dose Pharmacokinetics of Intravenous Morinidazole in Healthy Chinese Subjects
- PMID: 32152080
- PMCID: PMC7179596
- DOI: 10.1128/AAC.02067-19
Effects of Tenofovir on the Single-Dose Pharmacokinetics of Intravenous Morinidazole in Healthy Chinese Subjects
Abstract
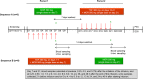
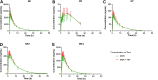
The effects of multiple-dose administration of tenofovir disoproxil fumarate (TDF) on the pharmacokinetics of morinidazole (MOR) were compared in healthy subjects. MOR exposure was similar, with an area under the curve from 0 h to infinity (AUC0-∞) treatment ratio for MOR+TDF/MOR of 1.01 (90% confidence interval, 0.97 to 1.06). No relevant differences were observed regarding plasma exposure of metabolites. Renal clearances of MOR and its metabolites were not affected by TDF. No unexpected safety or tolerability issues were observed.
Keywords: clinical pharmacokinetics; drug-drug interaction; morinidazole; organic anion transporter; tenofovir.
Copyright © 2020 Wu et al.
Figures

References
-
- Lv A. October 2008 Usage of alpha-(morpholin-1-base) methyl-2-methyl-5-azathio-1-alcohol in preparation of anti-trichomoniasis and anti-ameba medicines. China patent ZL 200510134254.3.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

