Visualizing the protons in a metalloenzyme electron proton transfer pathway
- PMID: 32152099
- PMCID: PMC7104402
- DOI: 10.1073/pnas.1918936117
Visualizing the protons in a metalloenzyme electron proton transfer pathway
Abstract
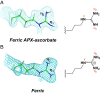
In redox metalloenzymes, the process of electron transfer often involves the concerted movement of a proton. These processes are referred to as proton-coupled electron transfer, and they underpin a wide variety of biological processes, including respiration, energy conversion, photosynthesis, and metalloenzyme catalysis. The mechanisms of proton delivery are incompletely understood, in part due to an absence of information on exact proton locations and hydrogen bonding structures in a bona fide metalloenzyme proton pathway. Here, we present a 2.1-Å neutron crystal structure of the complex formed between a redox metalloenzyme (ascorbate peroxidase) and its reducing substrate (ascorbate). In the neutron structure of the complex, the protonation states of the electron/proton donor (ascorbate) and all of the residues involved in the electron/proton transfer pathway are directly observed. This information sheds light on possible proton movements during heme-catalyzed oxygen activation, as well as on ascorbate oxidation.
Keywords: ascorbate; heme; neutron; peroxidase; proton transfer.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- Tsukihara T., et al. , The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 Å. Science 272, 1136–1144 (1996). - PubMed
-
- Iwata S., Ostermeier C., Ludwig B., Michel H., Structure at 2.8 Å resolution of cytochrome c oxidase from Paracoccus denitrificans. Nature 376, 660–669 (1995). - PubMed
-
- Buschmann S., et al. , The structure of cbb3 cytochrome oxidase provides insights into proton pumping. Science 329, 327–330 (2010). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

