Head-to-head comparison of in-house produced CD19 CAR-T cell in ALL and NHL patients
- PMID: 32152221
- PMCID: PMC7061891
- DOI: 10.1136/jitc-2019-000148
Head-to-head comparison of in-house produced CD19 CAR-T cell in ALL and NHL patients
Abstract
Background: CD19 chimeric antigen receptor T (CAR-T) cells demonstrate remarkable remission rates in pediatric and adult patients with refractory or relapsed (r/r) acute lymphoblastic leukemia (ALL) and non-Hodgkin's lymphoma (NHL). In 2016, we initiated a clinical trial with in-house produced CD19 CAR-T cells with a CD28 co-stimulatory domain. We analyzed, for the first time, differences in production features and phenotype between ALL and NHL patients.
Methods: Non-cryopreserved CAR-T cells were produced from patients' peripheral blood mononuclear cells within 9 to 10 days. 93 patients with r/r ALL and NHL were enrolled under the same study. CAR-T cells of ALL and NHL patients were produced simultaneously, allowing the head-to-head comparison.
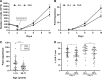
Results: All patients were heavily pretreated. Three patients dropped out from the study due to clinical deterioration (n=2) or production failure (n=1). Cells of ALL patients (n=37) expanded significantly better and contained more CAR-T cells than of NHL patients (n=53). Young age had a positive impact on the proliferation capacity. The infusion products from ALL patients contained significantly more naïve CAR-T cells and a significantly higher expression of the chemokine receptor CXCR3. PD-1, LAG-3, TIM-3, and CD28 were equally expressed. 100% of ALL patients and 94% of NHL patients received the target dose of 1×10e6 CAR-T/kg. The overall response rate was 84% (30/36) in ALL and 62% (32/52) in NHL. We further compared CAR-T cell infusion products to tumor infiltrating lymphocytes (TIL), another common type of T cell therapy, mainly clinically effective in solid tumors. CAR-T cells contained significantly more naïve T cells and central memory T cells and significantly less CCR5 compared to TIL infusion products.
Conclusions: The in-house production of CAR-T cells is highly efficient and fast. Clinical response rate is high. CAR-T cells can be successfully produced for 99% of patients in just 9 to 10 days. Cells derived from ALL patients demonstrate a higher proliferation rate and contain higher frequencies of CAR-T cells and naïve T cells than of NHL patients. In addition, understanding the differences between CAR-T and TIL infusion products, may provide an angle to develop CAR-T cells for the treatment of solid tumors in the future.
Trial registration number: ClinicalTrials.gov; CAR-T: NCT02772198, First posted: May 13, 2016; TIL: NCT00287131, First posted: February 6, 2006.
Keywords: cell engineering; hematologic neoplasms; immunotherapy; t-lymphocytes; tumours.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

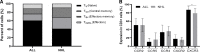
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
