Multiplexed CRISPR technologies for gene editing and transcriptional regulation
- PMID: 32152313
- PMCID: PMC7062760
- DOI: 10.1038/s41467-020-15053-x
Multiplexed CRISPR technologies for gene editing and transcriptional regulation
Abstract
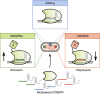
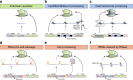
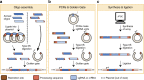
Multiplexed CRISPR technologies, in which numerous gRNAs or Cas enzymes are expressed at once, have facilitated powerful biological engineering applications, vastly enhancing the scope and efficiencies of genetic editing and transcriptional regulation. In this review, we discuss multiplexed CRISPR technologies and describe methods for the assembly, expression and processing of synthetic guide RNA arrays in vivo. Applications that benefit from multiplexed CRISPR technologies, including cellular recorders, genetic circuits, biosensors, combinatorial genetic perturbations, large-scale genome engineering and the rewiring of metabolic pathways, are highlighted. We also offer a glimpse of emerging challenges and emphasize experimental considerations for future studies.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

