Toward targeting inflammasomes: insights into their regulation and activation
- PMID: 32152420
- PMCID: PMC7118104
- DOI: 10.1038/s41422-020-0295-8
Toward targeting inflammasomes: insights into their regulation and activation
Abstract
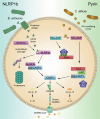
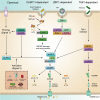
Inflammasomes are multi-component signaling complexes critical to the initiation of pyroptotic cell death in response to invading pathogens and cellular damage. A number of innate immune receptors have been reported to serve as inflammasome sensors. Activation of these sensors leads to the proteolytic activation of caspase-1, a proinflammatory caspase responsible for the cleavage of proinflammatory cytokines interleukin-1β and interleukin-18 and the effector of pyroptotic cell death, gasdermin D. Though crucial to the innate immune response to infection, dysregulation of inflammasome activation can lead to the development of inflammatory diseases, neurodegeneration, and cancer. Therefore, clinical interest in the modulation of inflammasome activation is swiftly growing. As such, it is imperative to develop a mechanistic understanding of the regulation of these complexes. In this review, we divide the regulation of inflammasome activation into three parts. We discuss the transcriptional regulation of inflammasome components and related proteins, the post-translational mechanisms of inflammasome activation, and advances in the understanding of the structural basis of inflammasome activation.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Janowski, A. M., Sutterwala, F. S. A typical inflammasomes. In: Di Virgilio, F., Pelegrín, P., eds. NLR Proteins: Methods and Protocols. Methods in Molecular Biology. New York, NY: Springer New York; 2016:45–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

