Inflammation in acquired hydrocephalus: pathogenic mechanisms and therapeutic targets
- PMID: 32152460
- PMCID: PMC7375440
- DOI: 10.1038/s41582-020-0321-y
Inflammation in acquired hydrocephalus: pathogenic mechanisms and therapeutic targets
Abstract
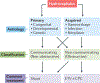
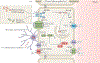
Hydrocephalus is the most common neurosurgical disorder worldwide and is characterized by enlargement of the cerebrospinal fluid (CSF)-filled brain ventricles resulting from failed CSF homeostasis. Since the 1840s, physicians have observed inflammation in the brain and the CSF spaces in both posthaemorrhagic hydrocephalus (PHH) and postinfectious hydrocephalus (PIH). Reparative inflammation is an important protective response that eliminates foreign organisms, damaged cells and physical irritants; however, inappropriately triggered or sustained inflammation can respectively initiate or propagate disease. Recent data have begun to uncover the molecular mechanisms by which inflammation - driven by Toll-like receptor 4-regulated cytokines, immune cells and signalling pathways - contributes to the pathogenesis of hydrocephalus. We propose that therapeutic approaches that target inflammatory mediators in both PHH and PIH could address the multiple drivers of disease, including choroid plexus CSF hypersecretion, ependymal denudation, and damage and scarring of intraventricular and parenchymal (glia-lymphatic) CSF pathways. Here, we review the evidence for a prominent role of inflammation in the pathogenic mechanism of PHH and PIH and highlight promising targets for therapeutic intervention. Focusing research efforts on inflammation could shift our view of hydrocephalus from that of a lifelong neurosurgical disorder to that of a preventable neuroinflammatory condition.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS109358/NS/NINDS NIH HHS/United States
- R01 AI145057/AI/NIAID NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- R01 NS105633/NS/NINDS NIH HHS/United States
- DP1 HD086071/HD/NICHD NIH HHS/United States
- R01 HL082517/HL/NHLBI NIH HHS/United States
- RF1 AG053991/AG/NIA NIH HHS/United States
- RF1 NS110049/NS/NINDS NIH HHS/United States
- T32 GM007205/GM/NIGMS NIH HHS/United States
- R01 NS100366/NS/NINDS NIH HHS/United States
- RF1 AG057575/AG/NIA NIH HHS/United States
- R01 HD096693/HD/NICHD NIH HHS/United States
- R01 HD085853/HD/NICHD NIH HHS/United States
- I01 BX002889/BX/BLRD VA/United States
- R01 NS060801/NS/NINDS NIH HHS/United States
- R01 NS102589/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

