Validation of nomogram-revised risk index and comparison with other models for extranodal nasal-type NK/T-cell lymphoma in the modern chemotherapy era: indication for prognostication and clinical decision-making
- PMID: 32152465
- PMCID: PMC7787971
- DOI: 10.1038/s41375-020-0791-3
Validation of nomogram-revised risk index and comparison with other models for extranodal nasal-type NK/T-cell lymphoma in the modern chemotherapy era: indication for prognostication and clinical decision-making
Abstract
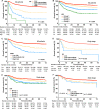
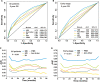
Derived from our original nomogram study by using the risk variables from multivariable analyses in the derivation cohort of 1383 patients with extranodal NK/T-cell lymphoma, nasal-type (ENKTCL) who were mostly treated with anthracycline-based chemotherapy, we propose an easily used nomogram-revised risk index (NRI), validated it and compared with Ann Arbor staging, the International Prognostic Index (IPI), Korean Prognostic Index (KPI), and prognostic index of natural killer lymphoma (PINK) for overall survival (OS) prediction by examining calibration, discrimination, and decision curve analysis in a validation cohort of 1582 patients primarily treated with non-anthracycline-based chemotherapy. The calibration of the NRI showed satisfactory for predicting 3- and 5-year OS in the validation cohort. The Harrell's C-index and integrated Brier score (IBS) of the NRI for OS prediction demonstrated a better performance than that of the Ann Arbor staging system, IPI, KPI, and PINK. Decision curve analysis of the NRI also showed a superior outcome. The NRI is a promising tool for stratifying patients with ENKTCL into risk groups for designing clinical trials and for selecting appropriate individualized treatment.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Au WY, Weisenburger DD, Intragumtornchai T, Nakamura S, Kim WS, Sng I, et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 2009;113:3931–7. doi: 10.1182/blood-2008-10-185256. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

