Lactic acidosis induces resistance to the pan-Akt inhibitor uprosertib in colon cancer cells
- PMID: 32152504
- PMCID: PMC7188671
- DOI: 10.1038/s41416-020-0777-y
Lactic acidosis induces resistance to the pan-Akt inhibitor uprosertib in colon cancer cells
Abstract
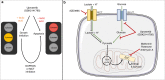
Background: Akt signalling regulates glycolysis and drives the Warburg effect in cancer, thus decreased glucose utilisation is a pharmacodynamic marker of Akt inhibition. However, cancer cells can utilise alternative nutrients to glucose for energy such as lactate, which is often elevated in tumours together with increased acidity. We therefore hypothesised that lactic acidosis may confer resistance to Akt inhibition.
Methods: The effect of the pan-Akt inhibitor uprosertib (GSK2141795), on HCT116 and LS174T colon cancer cells was evaluated in the presence and absence of lactic acid in vitro. Expression of downstream Akt signalling proteins was determined using a phosphokinase array and immunoblotting. Metabolism was assessed using 1H nuclear magnetic resonance spectroscopy, stable isotope labelling and gas chromatography-mass spectrometry.
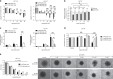
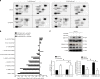
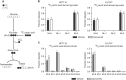
Results: Lactic acid-induced resistance to uprosertib was characterised by increased cell survival and reduced apoptosis. Uprosertib treatment reduced Akt signalling and glucose uptake irrespective of lactic acid supplementation. However, incorporation of lactate carbon and enhanced respiration was maintained in the presence of uprosertib and lactic acid. Inhibiting lactate transport or oxidative phosphorylation was sufficient to potentiate apoptosis in the presence of uprosertib.
Conclusions: Lactic acidosis confers resistance to uprosertib, which can be reversed by inhibiting lactate transport or oxidative metabolism.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Hirayama A, Kami K, Sugimoto M, Sugawara M, Toki N, Onozuka H, et al. Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry. Cancer Res. 2009;69:4918–4925. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

