Antibiotics in the clinical pipeline in October 2019
- PMID: 32152527
- PMCID: PMC7223789
- DOI: 10.1038/s41429-020-0291-8
Antibiotics in the clinical pipeline in October 2019
Abstract
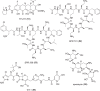
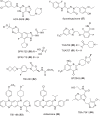
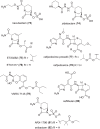
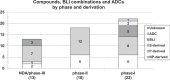
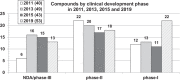
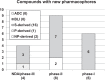
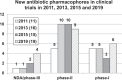
The development of new and effective antibacterial drugs to treat multi-drug resistant (MDR) bacteria, especially Gram-negative (G-ve) pathogens, is acknowledged as one of the world's most pressing health issues; however, the discovery and development of new, nontoxic antibacterials is not a straightforward scientific task, which is compounded by a challenging economic model. This review lists the antibacterials, β-lactamase/β-lactam inhibitor (BLI) combinations, and monoclonal antibodies (mAbs) first launched around the world since 2009 and details the seven new antibiotics and two new β-lactam/BLI combinations launched since 2016. The development status, mode of action, spectra of activity, lead source, and administration route for the 44 small molecule antibacterials, eight β-lactamase/BLI combinations, and one antibody drug conjugate (ADC) being evaluated in worldwide clinical trials at the end of October 2019 are described. Compounds discontinued from clinical development since 2016 and new antibacterial pharmacophores are also reviewed. There has been an increase in the number of early stage clinical candidates, which has been fueled by antibiotic-focused funding agencies; however, there is still a significant gap in the pipeline for the development of new antibacterials with activity against β-metallolactamases, orally administered with broad spectrum G-ve activity, and new treatments for MDR Acinetobacter and gonorrhea.
Conflict of interest statement
DLP has received grants and/or personal fees from Achaogen, AstraZeneca, Bayer, Cubist, Entasis Therapeutics, GlaxoSmithKline, Leo Pharmaceuticals, Merck Sharp & Dohme (Merck & Co), Pfizer, and Shionogi & Co. MSB declares no conflicts of interest.
Figures


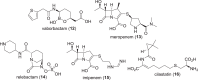
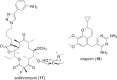

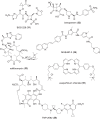
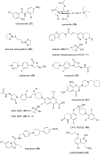


References
-
- Butler MS, Blaskovich MAT, Cooper MA. Antibiotics in the clinical pipeline at the end of 2015. J Antibiot. 2017;70:3–24. - PubMed
-
- Butler MS, Blaskovich MA, Cooper MA. Antibiotics in the clinical pipeline in 2013. J Antibiot. 2013;66:571–91. - PubMed
-
- Butler MS, Cooper MA. Antibiotics in the clinical pipeline in 2011. J Antibiot. 2011;64:413–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

