The Restrictome of Flaviviruses
- PMID: 32152893
- PMCID: PMC7462949
- DOI: 10.1007/s12250-020-00208-3
The Restrictome of Flaviviruses
Abstract
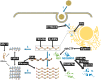
Flaviviruses are a genus of mostly arthropod-borne RNA viruses that cause a range of pathologies in humans. Basic knowledge on flaviviruses is rapidly expanding, partly due to their status as frequent emerging or re-emerging pathogens. Flaviviruses include the dengue, Zika, West Nile, tick-borne encephalitis and yellow fever viruses (DENV, ZIKV, WNV, TBEV and YFV, respectively). As is the case with other families of viruses, the success of productive infection of human cells by flaviviruses depends in part on the antiviral activity of a heterogeneous group of cellular antiviral proteins called restriction factors. Restriction factors are the effector proteins of the cell-autonomous innate response against viruses, an immune pathway that also includes virus sensors as well as intracellular and extracellular signal mediators such as type I interferons (IFN-I). In this review, I summarize recent progress toward the identification and characterization of flavivirus restriction factors. In particular, I focus on IFI6, Schlafen 11, FMRP, OAS-RNase L, RyDEN, members of the TRIM family of proteins (TRIM5α, TRIM19, TRIM56, TRIM69 and TRIM79α) and a new mechanism of action proposed for viperin. Recent and future studies on this topic will lead to a more complete picture of the flavivirus restrictome, defined as the ensemble of cellular factors with demonstrated anti-flaviviral activity.
Keywords: Flavivirus; Innate immunity; Interferon; Restriction factor; TRIM proteins.
Conflict of interest statement
The author declares that he has no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

