Regulatory T-cells in helminth infection: induction, function and therapeutic potential
- PMID: 32153025
- PMCID: PMC7341546
- DOI: 10.1111/imm.13190
Regulatory T-cells in helminth infection: induction, function and therapeutic potential
Abstract
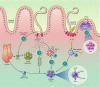
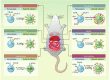
Helminth parasites infect an alarmingly large proportion of the world's population, primarily within tropical regions, and their ability to down-modulate host immunity is key to their persistence. Helminths have developed multiple mechanisms that induce a state of hyporesponsiveness or immune suppression within the host; of particular interest are mechanisms that drive the induction of regulatory T-cells (Tregs). Helminths actively induce Tregs either directly by secreting factors, such as the TGF-β mimic Hp-TGM, or indirectly by interacting with bystander cell types such as dendritic cells and macrophages that then induce Tregs. Expansion of Tregs not only enhances parasite survival but, in cases such as filarial infection, Tregs also play a role in preventing parasite-associated pathologies. Furthermore, Tregs generated during helminth infection have been associated with suppression of bystander immunopathologies in a range of inflammatory conditions such as allergy and autoimmune disease. In this review, we discuss evidence from natural and experimental infections that point to the pathways and molecules involved in helminth Treg induction, and postulate how parasite-derived molecules and/or Tregs might be applied as anti-inflammatory therapies in the future.
Keywords: immune regulation; immunomodulators; inflammation; therapy.
© 2020 The Authors. Immunology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare having no competing interests.
Figures
References
-
- James S, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N et al Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392:1789–858. - PMC - PubMed
-
- Schaub B, Lauener R, von Mutius E. The many faces of the hygiene hypothesis. J Allergy Clin Immunol 2006; 117:969–77; quiz 78. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

