Altered Rich-Club Organization and Regional Topology Are Associated With Cognitive Decline in Patients With Frontal and Temporal Gliomas
- PMID: 32153374
- PMCID: PMC7047345
- DOI: 10.3389/fnhum.2020.00023
Altered Rich-Club Organization and Regional Topology Are Associated With Cognitive Decline in Patients With Frontal and Temporal Gliomas
Abstract
Objectives: Gliomas are widely considered to be related to the altered topological organization of functional networks before operations. Tumors are usually thought to cause multimodal cognitive impairments. The structure is thought to form the basics of function, and the aim of this study was to reveal the rich-club organization and topological patterns of white matter (WM) structural networks associated with cognitive impairments in patients with frontal and temporal gliomas.
Methods: Graph theory approaches were utilized to reveal the global and regional topological organization and rich-club organization of WM structural networks of 14 controls (CN), 13 frontal tumors (FTumor), and 18 temporal tumors (TTumor). Linear regression was used to assess the relationship between cognitive performances and altered topological parameters.
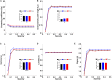
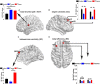
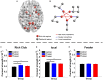
Results: When compared with CN, both FTumor and TTumor showed no alterations in small-world properties and global network efficiency, but instead showed altered local network efficiency. Second, FTumor and TTumor patients showed similar deficits in the nodal shortest path in the left rolandic operculum and degree centrality (DC) of the right dorsolateral and medial superior frontal gyrus (SFGmed). Third, compared to FTumor patients, TTumor patients showed a significantly higher DC in the right dorsolateral and SFGmed, a higher level of betweenness in the right SFGmed, and higher nodal efficiency in the left middle frontal gyrus and right SFGmed. Finally, rich-club organization was disrupted, with increased structural connectivity among rich-club nodes and reduced structural connectivity among peripheral nodes in FTumor and TTumor patients. Altered local efficiency in TTumor correlated with memory function, while altered local efficiency in FTumor correlated with the information processing speed.
Conclusion: Both FTumor and TTumor presented an intact global topology and altered regional topology related to cognitive impairment and may also share the convergent and divergent regional topological organization of WM structural networks. This suggested that a compensatory mechanism plays a key role in global topology formation in both FTumor and TTumor patients, and as such, development of a structural connectome for patients with brain tumors would be an invaluable medical resource and allow clinicians to make comprehensive preoperative planning.
Keywords: cognitive impairment; frontal tumors; rich-club organization; structural network; temporal tumors; topological organization.
Copyright © 2020 Liu, Yang, Hu, Xiao, Rao, Li, Liu, Zou, Chen and Liu.
Figures




References
-
- Bai F., Shu N., Yuan Y., Shi Y., Yu H., Wu D., et al. (2012). Topologically convergent and divergent structural connectivity patterns between patients with remitted geriatric depression and amnestic mild cognitive impairment. J. Neurosci. 32 4307–4318. 10.1523/JNEUROSCI.5061-11.2012 - DOI - PMC - PubMed
-
- Bhardwaj R. D., Mahmoodabadi S. Z., Otsubo H., Snead O. C., III, Rutka J. T., Widjaja E. (2010). Diffusion tensor tractography detection of functional pathway for the spread of epileptiform activity between temporal lobe and Rolandic region. Childs Nerv. Syst. 26 185–190. 10.1007/s00381-009-1017-1 - DOI - PubMed
LinkOut - more resources
Full Text Sources

