The Impact of ackA, pta, and ackA-pta Mutations on Growth, Gene Expression and Protein Acetylation in Escherichia coli K-12
- PMID: 32153530
- PMCID: PMC7047895
- DOI: 10.3389/fmicb.2020.00233
The Impact of ackA, pta, and ackA-pta Mutations on Growth, Gene Expression and Protein Acetylation in Escherichia coli K-12
Abstract
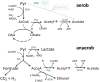
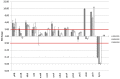
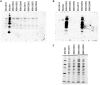
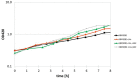
Acetate is a characteristic by-product of Escherichia coli K-12 growing in batch cultures with glucose, both under aerobic as well as anaerobic conditions. While the reason underlying aerobic acetate production is still under discussion, during anaerobic growth acetate production is important for ATP generation by substrate level phosphorylation. Under both conditions, acetate is produced by a pathway consisting of the enzyme phosphate acetyltransferase (Pta) producing acetyl-phosphate from acetyl-coenzyme A, and of the enzyme acetate kinase (AckA) producing acetate from acetyl-phosphate, a reaction that is coupled to the production of ATP. Mutants in the AckA-Pta pathway differ from each other in the potential to produce and accumulate acetyl-phosphate. In the publication at hand, we investigated different mutants in the acetate pathway, both under aerobic as well as anaerobic conditions. While under aerobic conditions only small changes in growth rate were observed, all acetate mutants showed severe reduction in growth rate and changes in the by-product pattern during anaerobic growth. The AckA- mutant showed the most severe growth defect. The glucose uptake rate and the ATP concentration were strongly reduced in this strain. This mutant exhibited also changes in gene expression. In this strain, the atoDAEB operon was significantly upregulated under anaerobic conditions hinting to the production of acetoacetate. During anaerobic growth, protein acetylation increased significantly in the ackA mutant. Acetylation of several enzymes of glycolysis and central metabolism, of aspartate carbamoyl transferase, methionine synthase, catalase and of proteins involved in translation was increased. Supplementation of methionine and uracil eliminated the additional growth defect of the ackA mutant. The data show that anaerobic, fermentative growth of mutants in the AckA-Pta pathway is reduced but still possible. Growth reduction can be explained by the lack of an important ATP generating pathway of mixed acid fermentation. An ackA deletion mutant is more severely impaired than pta or ackA-pta deletion mutants. This is most probably due to the production of acetyl-P in the ackA mutant, leading to increased protein acetylation.
Keywords: acetate metabolism; acetyl-P; fermentation; overflow; protein acetylation.
Copyright © 2020 Schütze, Benndorf, Püttker, Kohrs and Bettenbrock.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

