Pf Bacteriophage and Their Impact on Pseudomonas Virulence, Mammalian Immunity, and Chronic Infections
- PMID: 32153575
- PMCID: PMC7047154
- DOI: 10.3389/fimmu.2020.00244
Pf Bacteriophage and Their Impact on Pseudomonas Virulence, Mammalian Immunity, and Chronic Infections
Abstract
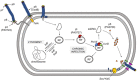
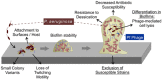
Pf bacteriophage are temperate phages that infect the bacterium Pseudomonas aeruginosa, a major cause of chronic lung infections in cystic fibrosis (CF) and other settings. Pf and other temperate phages have evolved complex, mutualistic relationships with their bacterial hosts that impact both bacterial phenotypes and chronic infection. We and others have reported that Pf phages are a virulence factor that promote the pathogenesis of P. aeruginosa infections in animal models and are associated with worse skin and lung infections in humans. Here we review the biology of Pf phage and what is known about its contributions to pathogenesis and clinical disease. First, we review the structure, genetics, and epidemiology of Pf phage. Next, we address the diverse and surprising ways that Pf phages contribute to P. aeruginosa phenotypes including effects on biofilm formation, antibiotic resistance, and motility. Then, we cover data indicating that Pf phages suppress mammalian immunity at sites of bacterial infection. Finally, we discuss recent literature implicating Pf in chronic P. aeruginosa infections in CF and other settings. Together, these reports suggest that Pf bacteriophage have direct effects on P. aeruginosa infections and that temperate phages are an exciting frontier in microbiology, immunology, and human health.
Keywords: Inovirus; Pf phage; Pseudomonas aeruginosa; bacteriophage; cystic fibrosis; immunology; infection; wound.
Copyright © 2020 Secor, Burgener, Kinnersley, Jennings, Roman-Cruz, Popescu, Van Belleghem, Haddock, Copeland, Michaels, de Vries, Chen, Pourtois, Wheeler, Milla and Bollyky.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

