Crosstalk Between Gut Microbiota and Innate Immunity and Its Implication in Autoimmune Diseases
- PMID: 32153586
- PMCID: PMC7047319
- DOI: 10.3389/fimmu.2020.00282
Crosstalk Between Gut Microbiota and Innate Immunity and Its Implication in Autoimmune Diseases
Abstract
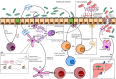
The emerging concept of microbiota contributing to local mucosal homeostasis has fueled investigation into its specific role in immunology. Gut microbiota is mostly responsible for maintaining the balance between host defense and immune tolerance. Dysbiosis of gut microbiota has been shown to be related to various alterations of the immune system. This review focuses on the reciprocal relationship between gut microbiota and innate immunity compartment, with emphasis on gut-associated lymphoid tissue, innate lymphoid cells, and phagocytes. From a clinical perspective, the review gives a possible explanation of how the "gut microbiota-innate immunity" axis might contribute to the pathogenesis of autoimmune diseases like rheumatoid arthritis, spondyloarthritis, and systemic lupus erythematosus.
Keywords: gut microbiota; innate immunity; innate lymphoid cells; rheumatoid arthritis; spondyloarthritis; systemic lupus erythematosus.
Copyright © 2020 Jiao, Wu, Huntington and Zhang.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

