Effects of dexrazoxane on doxorubicin-related cardiotoxicity and second malignant neoplasms in children with osteosarcoma: a report from the Children's Oncology Group
- PMID: 32154021
- PMCID: PMC7048050
- DOI: 10.1186/s40959-019-0050-9
Effects of dexrazoxane on doxorubicin-related cardiotoxicity and second malignant neoplasms in children with osteosarcoma: a report from the Children's Oncology Group
Abstract
Background: Dexrazoxane protects from lower-cumulative-dose doxorubicin cardiotoxicity, but the effect of dexrazoxane in children with sarcoma treated with higher-cumulative-dose doxorubicin is unknown.
Methods: We evaluated children with osteosarcoma (OS) on two Children's Oncology Group trials with higher dose doxorubicin (375-600 mg/m2) preceded by dexrazoxane (10:1 dexrazoxane:doxorubicin dosing). They were evaluated after the minimum expected treatment time (METT), defined as 28 weeks. Cardiotoxicity was identified by echocardiography and serum N-terminal pro-brain natriuretic peptide (NT-proBNP). Second malignant neoplasm (SMN) data was collected.
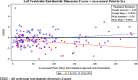
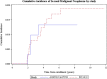
Results: All children had normal left ventricular (LV) systolic function as measured by LV fractional shortening and no heart failure. The end-diastolic septal thickness Z-scores (P < 0.01) and LV mass Z-scores (P < 0.01) were significantly smaller than normal for body-surface area in both sexes. The average LV mass Z-scores were significantly smaller for girls (P < 0.01) and marginally smaller for boys (P = 0.06). Girls had significantly smaller LV end-diastolic dimension Z-scores normalized to BSA (P < 0.01) compared to healthy controls and had significant increases in NT-proBNP. Four children developed SMNs as first events, a rate similar to historical controls.
Conclusions: Dexrazoxane prevented LV dysfunction and heart failure in children with OS receiving higher dose doxorubicin. However, LV structural changes were not fully prevented, especially in girls. As a result, hearts become abnormally small for body size, resulting in higher LV stress. Dexrazoxane did not increase the risk of SMN. Dexrazoxane should be used in this population, particularly for girls, to mitigate anthracycline-induced cardiotoxicity.
Trial registrations: ClinicalTrials.gov: NCT00003937 (P9754) registered 1 Nov 1999, and NCT00023998 (AOST0121) registered 13 Sept 2001.
Keywords: Cardiotoxicity; Doxorubicin; Osteosarcoma; Pediatrics.
© The Author(s). 2019.
Conflict of interest statement
Competing interestsL.K. is an employee of the contract research organization Covance. M.K. is a consultant for Merck. K.J. has received travel reimbursement from Loxo oncology. R.G. has received laboratory research funding from Eisai. N.M. is an employee of Five Prime Therapeutics. P.M. has received travel reimbursement from Takeda and is a consultant for Eli Lilly and Astellas. S.L. is a consultant for Clinigen, a manufacturer of dexrazoxane, and Editor in Chief of Cardio-Oncology.
Figures


References
-
- Getz Kelly D., Sung Lillian, Ky Bonnie, Gerbing Robert B., Leger Kasey J., Leahy Allison Barz, Sack Leah, Woods William G., Alonzo Todd, Gamis Alan, Aplenc Richard. Occurrence of Treatment-Related Cardiotoxicity and Its Impact on Outcomes Among Children Treated in the AAML0531 Clinical Trial: A Report From the Children’s Oncology Group. Journal of Clinical Oncology. 2019;37(1):12–21. doi: 10.1200/JCO.18.00313. - DOI - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
