Current Treatment and Outcomes Benchmark for Locally Advanced or Metastatic Urothelial Cancer From a Large UK-Based Single Centre
- PMID: 32154169
- PMCID: PMC7044411
- DOI: 10.3389/fonc.2020.00167
Current Treatment and Outcomes Benchmark for Locally Advanced or Metastatic Urothelial Cancer From a Large UK-Based Single Centre
Abstract
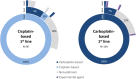
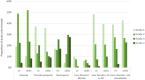
Objectives: To characterize treatment patterns and survival outcomes for patients with locally advanced or metastatic malignancy of the urothelial tract during a period immediately preceding the widespread use of immune checkpoint inhibitors in the UK. Patients and Methods: We retrospectively examined the electronic case notes of patients attending the Leeds Cancer Center, UK with locally advanced or metastatic urothelial carcinoma, receiving chemotherapy between January 2003 and March 2017. Patient characteristics, treatment patterns, and outcomes were collected. Summary and descriptive statistics were calculated for categorical and continuous variables as appropriate. The Kaplan-Meier method was used to estimate median survival and Cox regression proportional hazards model was used to explore relationships between clinical variables and outcome. Results: Two hundred and sixteen patients made up the study cohort, with a median age of 66 years (range: 35-83) and 72.7% being male. First-line treatment consisted of either a cisplatin- (44%) or carboplatin-based regimen (48%) in the majority of patients. Twenty seven percent of patients received a second-line of treatment (most commonly single-agent paclitaxel) following a first-line platinum containing regimen. Grade 4 neutropenia was observed in 19 and 27% of those treated with a first-line cisplatin- and carboplatin-based regimen, respectively. The median overall survival (mOS) of the study cohort was estimated to be 16.2 months (IQR: 10.6-28.3 months). Receipt by patients of cisplatin-based chemotherapy was associated with a longer mOS and this association persisted when survival analysis was adjusted for age, sex, performance status and presence of distant metastases. Conclusions: This study provides a useful benchmark for outcomes achieved in a real-world setting for patients with locally advanced or metastatic UC treated with chemotherapy in the immediate pre-immunotherapy era.
Keywords: carboplatin; chemotherapy; cisplatin; real-world; urothelial cancer.
Copyright © 2020 Cheeseman, Thompson, Sopwith, Godden, Seshagiri, Adedokun, Zucker, Jain, Kotwal, Prescott, Henry, Joseph, Chilka, Roulson, Weston, Burbidge, Brown, Jagdev, Ralph, Hall and Vasudev.
Figures


References
-
- Cancer Registration Statistics, England. Office for National Statistics. Available online at: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/... (accessed September 3, 2018).
-
- Bladder Cancer Statistics. Cancer Research UK (2015). Available online at: https://www.cancerresearchuk.org/health-professional/cancer-statistics/s... (accessed December 13, 2018).
-
- von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. (2000) 18:3068–77. 10.1200/JCO.2000.18.17.3068 - DOI - PubMed
LinkOut - more resources
Full Text Sources

