Auto Micro Atomization Delivery of Human Epidermal Organoids Improves Therapeutic Effects for Skin Wound Healing
- PMID: 32154237
- PMCID: PMC7046802
- DOI: 10.3389/fbioe.2020.00110
Auto Micro Atomization Delivery of Human Epidermal Organoids Improves Therapeutic Effects for Skin Wound Healing
Abstract
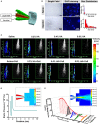
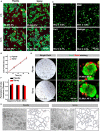
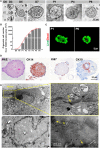
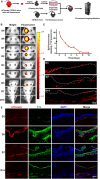
Severe skin wounds are often associated with large areas of damaged tissue, resulting in substantial loss of fluids containing electrolytes and proteins. The net result is a vulnerability clinically to skin infections. Therapies aiming to close these large openings are effective in reducing the complications of severe skin wounds. Recently, cell transplantation therapy showed the potential for rapid re-epithelialization of severe skin wounds. Here, we show the improved effects of cell transplantation therapy using a robust protocol of efficient expansion and delivery of epidermal cells for treatment of severe skin wounds. Human skin tissues were used to generate human epidermal organoids maintained under newly established culture conditions. The human epidermal organoids showed an improved capacity of passaging for at least 10 rounds, enabling organoids to expand to cell numbers required for clinical applications. A newly designed auto micro-atomization device (AMAD) was developed for delivery of human epidermal organoids onto the sites of severe skin wounds enhancing uniform and concentrated delivery of organoids, facilitating their engraftment and differentiation for skin reconstitution. With the optimal design and using pneumatic AMAD, both survival and functions of organoids were effectively protected during the spraying process. Cells in the sprayed human epidermal organoids participated in the regeneration of the epidermis at wound sites in a mouse model and accelerated wound healing significantly. The novel AMAD and out new protocol with enhanced effects with respect to both organoid expansion and efficient transplantation will be used for clincal treatments of complex, uneven, or large-area severe skin wounds.
Keywords: auto micro atomization device (AMAD); cell delivery; cell therapy; organoids; wound healing.
Copyright © 2020 Chang, Liu, Guo, Fang, Wang, Wang, Liu, Reid and Wang.
Figures


References
-
- Bahoric A., Harrop A. R., Clarke H. M., Zuker R. M. (1997). Aerosol vehicle for delivery of epidermal cells – an in vitro study. Can. J. Plast. Surg. 5, 153–156. 10.1177/229255039700500301 - DOI
LinkOut - more resources
Full Text Sources

