Upregulation of PD-L1 Expression by Prostaglandin E2 and the Enhancement of IFN-γ by Anti-PD-L1 Antibody Combined With a COX-2 Inhibitor in Mycoplasma bovis Infection
- PMID: 32154274
- PMCID: PMC7045061
- DOI: 10.3389/fvets.2020.00012
Upregulation of PD-L1 Expression by Prostaglandin E2 and the Enhancement of IFN-γ by Anti-PD-L1 Antibody Combined With a COX-2 Inhibitor in Mycoplasma bovis Infection
Abstract
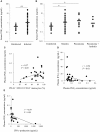
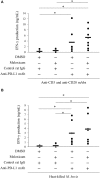
Bovine mycoplasmosis caused by Mycoplasma bovis results in pneumonia and mastitis in cattle. We previously demonstrated that the programmed death 1 (PD-1)/PD-ligand 1 (PD-L1) pathway is involved in immune dysfunction during M. bovis infection and that prostaglandin E2 (PGE2) suppressed immune responses and upregulated PD-L1 expression in Johne's disease, a bacterial infection in cattle. In this study, we investigated the role of PGE2 in immune dysfunction and the relationship between PGE2 and the PD-1/PD-L1 pathway in M. bovis infection. In vitro stimulation with M. bovis upregulated the expressions of PGE2 and PD-L1 presumably via Toll-like receptor 2 in bovine peripheral blood mononuclear cells (PBMCs). PGE2 levels of peripheral blood in infected cattle were significantly increased compared with those in uninfected cattle. Remarkably, plasma PGE2 levels were positively correlated with the proportions of PD-L1+ monocytes in M. bovis-infected cattle. Additionally, plasma PGE2 production in infected cattle was negatively correlated with M. bovis-specific interferon (IFN)-γ production from PBMCs. These results suggest that PGE2 could be one of the inducers of PD-L1 expression and could be involved in immunosuppression during M. bovis infection. In vitro blockade assays using anti-bovine PD-L1 antibody and a cyclooxygenase 2 inhibitor significantly upregulated the M. bovis-specific IFN-γ response. Our study findings might contribute to the development of novel therapeutic strategies for bovine mycoplasmosis that target PGE2 and the PD-1/PD-L1 pathway.
Keywords: Mycoplasma bovis; PD-1; PD-L1; T-cell exhaustion; cattle; immune dysfunction; immunoinhibitory molecules; prostaglandin E2.
Copyright © 2020 Goto, Konnai, Hirano, Kohara, Okagawa, Maekawa, Sajiki, Watari, Minato, Kobayashi, Gondaira, Higuchi, Koiwa, Tajima, Taguchi, Uemura, Yamada, Kaneko, Kato, Yamamoto, Toda, Suzuki, Murata and Ohashi.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

