The mRNA-Binding Protein IGF2BP1 Restores Fetal Hemoglobin in Cultured Erythroid Cells from Patients with β-Hemoglobin Disorders
- PMID: 32154328
- PMCID: PMC7056608
- DOI: 10.1016/j.omtm.2020.01.011
The mRNA-Binding Protein IGF2BP1 Restores Fetal Hemoglobin in Cultured Erythroid Cells from Patients with β-Hemoglobin Disorders
Abstract
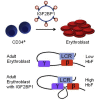
Sickle cell disease (SCD) and β-thalassemia are caused by structural abnormality or inadequate production of adult hemoglobin (HbA, α2β2), respectively. Individuals with either disorder are asymptomatic before birth because fetal hemoglobin (HbF, α2γ2) is unaffected. Thus, reversal of the switch from HbF to HbA could reduce or even prevent symptoms these disorders. In this study, we show that insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) is one factor that could accomplish this goal. IGF2BP1 is a fetal factor that undergoes a transcriptional switch consistent with the transition from HbF to HbA. Lentivirus delivery of IGF2BP1 to CD34+ cells of healthy adult donors reversed hemoglobin production toward the fetal type in culture-differentiated erythroid cells. Analogous studies using patient-derived CD34+ cells revealed that IGF2BP1-dependent HbF induction could ameliorate the chain imbalance in β-thalassemia or potently suppress expression of sickle β-globin in SCD. In all cases, fetal γ-globin mRNA increased and adult β-globin decreased due, in part, to formation of contacts between the locus control region (LCR) and γ-globin genes. We conclude that expression of IGF2BP1 in adult erythroid cells has the potential to maximize HbF expression in patients with severe β-hemoglobin disorders by reversing the developmental γ- to β-globin switch.
Keywords: IGF2BP1; beta-thalassemia; fetal hemoglobin; gene regulation; gene therapy; hemoglobinopathies; lentivirus; sickle cell disease.
© 2020 The Author(s).
Figures



Similar articles
-
Comparative analysis of lentiviral gene transfer approaches designed to promote fetal hemoglobin production for the treatment of β-hemoglobinopathies.Blood Cells Mol Dis. 2020 Sep;84:102456. doi: 10.1016/j.bcmd.2020.102456. Epub 2020 May 29. Blood Cells Mol Dis. 2020. PMID: 32498026
-
IGF2BP1 overexpression causes fetal-like hemoglobin expression patterns in cultured human adult erythroblasts.Proc Natl Acad Sci U S A. 2017 Jul 11;114(28):E5664-E5672. doi: 10.1073/pnas.1609552114. Epub 2017 Jun 26. Proc Natl Acad Sci U S A. 2017. PMID: 28652347 Free PMC article.
-
Understanding heterogeneity of fetal hemoglobin induction through comparative analysis of F and A erythroblasts.Blood. 2020 May 28;135(22):1957-1968. doi: 10.1182/blood.2020005058. Blood. 2020. PMID: 32268371 Free PMC article.
-
Fetal hemoglobin chemical inducers for treatment of hemoglobinopathies.Ann Hematol. 2009 Jun;88(6):505-28. doi: 10.1007/s00277-008-0637-y. Epub 2008 Nov 15. Ann Hematol. 2009. PMID: 19011856 Review.
-
Disorders of the synthesis of human fetal hemoglobin.IUBMB Life. 2008 Feb;60(2):94-111. doi: 10.1002/iub.4. IUBMB Life. 2008. PMID: 18379999 Review.
Cited by
-
Stability selection enhances feature selection and enables accurate prediction of gestational age using only five DNA methylation sites.Clin Epigenetics. 2023 Jul 13;15(1):114. doi: 10.1186/s13148-023-01528-3. Clin Epigenetics. 2023. PMID: 37443060 Free PMC article.
-
Epigenetic Regulation of β-Globin Genes and the Potential to Treat Hemoglobinopathies through Epigenome Editing.Genes (Basel). 2023 Feb 25;14(3):577. doi: 10.3390/genes14030577. Genes (Basel). 2023. PMID: 36980849 Free PMC article. Review.
-
Decitabine-Driven Foetal Haemoglobin Induction in Townes Mice and Human Erythroblasts.EJHaem. 2025 Aug 4;6(4):e70120. doi: 10.1002/jha2.70120. eCollection 2025 Aug. EJHaem. 2025. PMID: 40761187 Free PMC article.
-
Comprehensive Characterization and Global Transcriptome Analysis of Human Fetal Liver Terminal Erythropoiesis.Genomics Proteomics Bioinformatics. 2023 Dec;21(6):1117-1132. doi: 10.1016/j.gpb.2023.07.001. Epub 2023 Aug 30. Genomics Proteomics Bioinformatics. 2023. PMID: 37657739 Free PMC article.
-
Exploratory Review and In Silico Insights into circRNA and RNA-Binding Protein Roles in γ-Globin to β-Globin Switching.Cells. 2025 Feb 19;14(4):312. doi: 10.3390/cells14040312. Cells. 2025. PMID: 39996784 Free PMC article. Review.
References
-
- Bunn H.F. Pathogenesis and treatment of sickle cell disease. N. Engl. J. Med. 1997;337:762–769. - PubMed
-
- Weatherall D.J. The thalassemias. In: Stamatoyannopoulos G., Majerus P., Perlmutter R., Varmus H.E., editors. The Molecular Basis for Blood Disorders. W.B. Saunders; 2001. pp. 183–226.
-
- Thein S.L., Menzel S. Discovering the genetics underlying foetal haemoglobin production in adults. Br. J. Haematol. 2009;145:455–467. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

