KLK6 expression in skin induces PAR1-mediated psoriasiform dermatitis and inflammatory joint disease
- PMID: 32155135
- PMCID: PMC7260032
- DOI: 10.1172/JCI133159
KLK6 expression in skin induces PAR1-mediated psoriasiform dermatitis and inflammatory joint disease
Abstract
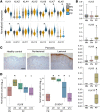
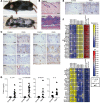
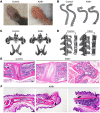
Kallikrein-related peptidase 6 (KLK6) is a secreted serine protease hypothesized to promote inflammation via cleavage of protease-activated receptor 1 (PAR1) and PAR2. KLK6 levels are elevated in multiple inflammatory and autoimmune conditions, but no definitive role in pathogenesis has been established. Here, we show that skin-targeted overexpression of KLK6 causes generalized, severe psoriasiform dermatitis with spontaneous development of debilitating psoriatic arthritis-like joint disease. The psoriatic skin and joint phenotypes are reversed by normalization of skin KLK6 levels and attenuated following genetic elimination of PAR1 but not PAR2. Conservation of this regulatory pathway was confirmed in human psoriasis using vorapaxar, an FDA-approved PAR1 antagonist, on explanted lesional skin from patients with psoriasis. Beyond defining a critical role for KLK6/PAR1 signaling in promoting psoriasis, our results demonstrate that KLK6/PAR1-mediated inflammation in the skin alone is sufficient to drive inflammatory joint disease. Further, we identify PAR1 as a promising cytokine-independent target in therapy of psoriasis and psoriatic arthritis.
Keywords: Arthritis; Dermatology; Inflammation; Kallikrein; Skin.
Conflict of interest statement
Figures

Comment in
-
Proteinase KLK6 links skin and joint inflammation in PsA.Nat Rev Rheumatol. 2020 May;16(5):250. doi: 10.1038/s41584-020-0416-2. Nat Rev Rheumatol. 2020. PMID: 32231304 No abstract available.
Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Etanercept and infliximab for the treatment of psoriatic arthritis: a systematic review and economic evaluation.Health Technol Assess. 2006 Sep;10(31):iii-iv, xiii-xvi, 1-239. doi: 10.3310/hta10310. Health Technol Assess. 2006. PMID: 16948890
-
Proteomics of skin proteins in psoriasis: from discovery and verification in a mouse model to confirmation in humans.Mol Cell Proteomics. 2015 Jan;14(1):109-19. doi: 10.1074/mcp.M114.042242. Epub 2014 Oct 28. Mol Cell Proteomics. 2015. PMID: 25351201 Free PMC article.
-
Critical role for PAR1 in kallikrein 6-mediated oligodendrogliopathy.Glia. 2013 Sep;61(9):1456-70. doi: 10.1002/glia.22534. Epub 2013 Jul 8. Glia. 2013. PMID: 23832758 Free PMC article.
Cited by
-
A KLK6 Activity-Based Probe Reveals a Role for KLK6 Activity in Pancreatic Cancer Cell Invasion.J Am Chem Soc. 2022 Dec 14;144(49):22493-22504. doi: 10.1021/jacs.2c07378. Epub 2022 Nov 22. J Am Chem Soc. 2022. PMID: 36413626 Free PMC article.
-
Insights into Nutritional Strategies in Psoriasis.Nutrients. 2023 Aug 10;15(16):3528. doi: 10.3390/nu15163528. Nutrients. 2023. PMID: 37630719 Free PMC article. Review.
-
Proteomic and Metabolomic Changes in Psoriasis Preclinical and Clinical Aspects.Int J Mol Sci. 2023 May 30;24(11):9507. doi: 10.3390/ijms24119507. Int J Mol Sci. 2023. PMID: 37298466 Free PMC article. Review.
-
Clinical Response Characteristics of Salivary Proteins in the Management Strategy of Diabetes-Associated Periodontitis.J Proteome Res. 2025 Mar 7;24(3):1161-1179. doi: 10.1021/acs.jproteome.4c00701. Epub 2025 Feb 26. J Proteome Res. 2025. PMID: 40008981
-
New Frontiers in Psoriatic Disease Research, Part I: Genetics, Environmental Triggers, Immunology, Pathophysiology, and Precision Medicine.J Invest Dermatol. 2021 Sep;141(9):2112-2122.e3. doi: 10.1016/j.jid.2021.02.764. Epub 2021 Jul 22. J Invest Dermatol. 2021. PMID: 34303522 Free PMC article. Review.
References
-
- Oikonomopoulou K, et al. Kallikrein-mediated cell signalling: targeting proteinase-activated receptors (PARs) Biol Chem. 2006;387(6):817–824. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR073196/AR/NIAMS NIH HHS/United States
- P30 AR039750/AR/NIAMS NIH HHS/United States
- UL1 TR002548/TR/NCATS NIH HHS/United States
- R01 AI172944/AI/NIAID NIH HHS/United States
- P30 AR075043/AR/NIAMS NIH HHS/United States
- R01 AI130025/AI/NIAID NIH HHS/United States
- P50 AR055508/AR/NIAMS NIH HHS/United States
- P50 AR070590/AR/NIAMS NIH HHS/United States
- T32 AR007197/AR/NIAMS NIH HHS/United States
- R01 AR063437/AR/NIAMS NIH HHS/United States
- R01 AR069071/AR/NIAMS NIH HHS/United States
- R01 AR062546/AR/NIAMS NIH HHS/United States
- R01 AR075777/AR/NIAMS NIH HHS/United States
- R21 AR063852/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

