A Preliminary Study of Cu Exposure Effects upon Alzheimer's Amyloid Pathology
- PMID: 32155778
- PMCID: PMC7175127
- DOI: 10.3390/biom10030408
A Preliminary Study of Cu Exposure Effects upon Alzheimer's Amyloid Pathology
Abstract
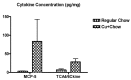
A large body of evidence indicates that dysregulation of cerebral biometals (Fe, Cu, Zn) and their interactions with amyloid precursor protein (APP) and Aβ amyloid may contribute to the Alzheimer's disease (AD) Aβ amyloid pathology. However, the molecular underpinnings associated with the interactions are still not fully understood. Herein we have further validated the exacerbation of Aβ oligomerization by Cu and H2O2 in vitro. We have also reported that Cu enhanced APP translations via its 5' untranslated region (5'UTR) of mRNA in SH-SY5Y cells, and increased Aβ amyloidosis and expression of associated pro-inflammatory cytokines such as MCP-5 in Alzheimer's APP/PS1 doubly transgenic mice. This preliminary study may further unravel the pathogenic role of Cu in Alzheimer's Aβ amyloid pathogenesis, warranting further investigation.
Keywords: Alzheimer’s disease; Aβ amyloid; amyloid precursor protein; copper; cytokine; neuroinflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Squitti R., Ghidoni R., Simonelli I., Ivanova I.D., Colabufo N.A., Zuin M., Benussi L., Binetti G., Cassetta E., Rongioletti M., et al. Copper dyshomeostasis in Wilson disease and Alzheimer’s disease as shown by serum and urine copper indicators. J. Trace Elem. Med. Biol. 2018;45:181–188. doi: 10.1016/j.jtemb.2017.11.005. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

