Clinical Significance of Cytoplasmic IgE-Positive Mast Cells in Eosinophilic Chronic Rhinosinusitis
- PMID: 32155995
- PMCID: PMC7084524
- DOI: 10.3390/ijms21051843
Clinical Significance of Cytoplasmic IgE-Positive Mast Cells in Eosinophilic Chronic Rhinosinusitis
Abstract
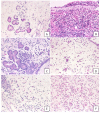
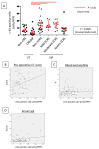
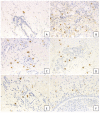
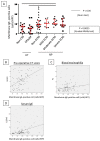
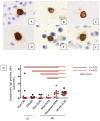
Cross-linking of antigen-specific IgE bound to the high-affinity IgE receptor (FcεRI) on the surface of mast cells with multivalent antigens results in the release of mediators and development of type 2 inflammation. FcεRI expression and IgE synthesis are, therefore, critical for type 2 inflammatory disease development. In an attempt to clarify the relationship between eosinophilic chronic rhinosinusitis (ECRS) and mast cell infiltration, we analyzed mast cell infiltration at lesion sites and determined its clinical significance. Mast cells are positive for c-kit, and IgE in uncinated tissues (UT) and nasal polyps (NP) were examined by immunohistochemistry. The number of positive cells and clinicopathological factors were analyzed. Patients with ECRS exhibited high levels of total IgE serum levels and elevated peripheral blood eosinophil ratios. As a result, the number of mast cells with membranes positive for c-kit and IgE increased significantly in lesions forming NP. Therefore, we classified IgE-positive mast cells into two groups: membrane IgE-positive cells and cytoplasmic IgE-positive cells. The amount of membrane IgE-positive mast cells was significantly increased in moderate ECRS. A positive correlation was found between the membrane IgE-positive cells and the radiological severity score, the ratio of eosinophils, and the total serum IgE level. The number of cytoplasmic IgE-positive mast cells was significantly increased in moderate and severe ECRS. A positive correlation was observed between the cytoplasmic IgE-positive cells and the radiological severity score, the ratio of eosinophils in the blood, and the total IgE level. These results suggest that the process of mast cell internalization of antigens via the IgE receptor is involved in ECRS pathogenesis.
Keywords: IgE; c-kit; eosinophilic chronic rhinosinusitis; mast cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Elevated MS4A2 and IgE interaction in nasal polyps contributing to poor postoperative prognosis in patients with eosinophilic chronic rhinosinusitis.Allergol Int. 2025 Apr;74(2):283-291. doi: 10.1016/j.alit.2024.10.008. Epub 2025 Jan 18. Allergol Int. 2025. PMID: 39828458
-
Distribution, subtype population, and IgE positivity of mast cells in chronic rhinosinusitis with nasal polyps.Ann Allergy Asthma Immunol. 2017 Aug;119(2):120-128. doi: 10.1016/j.anai.2017.05.019. Epub 2017 Jun 19. Ann Allergy Asthma Immunol. 2017. PMID: 28634018
-
Increased local IgE production induced by common aeroallergens and phenotypic alteration of mast cells in Chinese eosinophilic, but not non-eosinophilic, chronic rhinosinusitis with nasal polyps.Clin Exp Allergy. 2014;44(5):690-700. doi: 10.1111/cea.12304. Clin Exp Allergy. 2014. PMID: 24597471 Free PMC article.
-
Formation of nasal polyps: The roles of innate type 2 inflammation and deposition of fibrin.J Allergy Clin Immunol. 2020 Mar;145(3):740-750. doi: 10.1016/j.jaci.2020.01.027. J Allergy Clin Immunol. 2020. PMID: 32145873 Free PMC article. Review.
-
The role of human mast cell-derived cytokines in eosinophil biology.J Interferon Cytokine Res. 2004 May;24(5):271-81. doi: 10.1089/107999004323065057. J Interferon Cytokine Res. 2004. PMID: 15153310 Review.
Cited by
-
Involvement of CD40-CD40L and ICOS-ICOSL in the development of chronic rhinosinusitis by targeting eosinophils.Front Immunol. 2023 Jun 1;14:1171308. doi: 10.3389/fimmu.2023.1171308. eCollection 2023. Front Immunol. 2023. PMID: 37325657 Free PMC article.
-
Gαi1/3 signaling mediates IL-5-induced eosinophil activation and type 2 inflammation in eosinophilic chronic rhinosinusitis.Front Immunol. 2025 Jan 7;15:1460104. doi: 10.3389/fimmu.2024.1460104. eCollection 2024. Front Immunol. 2025. PMID: 39840047 Free PMC article.
-
Personalized Medicine in Chronic Rhinosinusitis: Treatable Traits Using Biologics for Unmet Needs.Allergy Asthma Immunol Res. 2025 Jan;17(1):8-21. doi: 10.4168/aair.2025.17.1.8. Allergy Asthma Immunol Res. 2025. PMID: 39895599 Free PMC article. Review.
-
Spotlight on a Short-Time Treatment with the IL-4/IL-13 Receptor Blocker in Patients with CRSwNP: microRNAs Modulations and Preliminary Clinical Evidence.Genes (Basel). 2022 Dec 15;13(12):2366. doi: 10.3390/genes13122366. Genes (Basel). 2022. PMID: 36553635 Free PMC article.
-
Phenotypic and Functional Diversity of Mast Cells.Int J Mol Sci. 2020 May 28;21(11):3835. doi: 10.3390/ijms21113835. Int J Mol Sci. 2020. PMID: 32481605 Free PMC article.
References
-
- Matsuwaki Y., Ookushi T., Asaka D., Mori E., Nakajima T., Yoshida T., Kojima J., Chiba S., Ootori N., Moriyama H. Chronic rhinosinusitis: Risk factors for the recurrence of chronic rhinosinusitis based on 5-year follow-up after endoscopic sinus surgery. Int. Arch. Allergy Immunol. 2008;146:77–81. doi: 10.1159/000126066. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

