Beneficial Propionibacteria within a Probiotic Emmental Cheese: Impact on Dextran Sodium Sulphate-Induced Colitis in Mice
- PMID: 32156075
- PMCID: PMC7142753
- DOI: 10.3390/microorganisms8030380
Beneficial Propionibacteria within a Probiotic Emmental Cheese: Impact on Dextran Sodium Sulphate-Induced Colitis in Mice
Abstract
Backgrounds and aims: Inflammatory Bowel Diseases (IBD), including Ulcerative Colitis (UC), coincide with alterations in the gut microbiota. Consumption of immunomodulatory strains of probiotic bacteria may induce or prolong remission in UC patients. Fermented foods, including cheeses, constitute major vectors for bacteria consumption. New evidences revealed anti-inflammatory effects in selected strains of Propionibacterium freudenreichii. We thus hypothesized that consumption of a functional cheese, fermented by such a strain, may exert a positive effect on IBD.
Methods: We investigated the impact of cheese fermented by P. freudenreichii on gut inflammation. We developed an experimental single-strain cheese solely fermented by a selected immunomodulatory strain of P. freudenreichii, CIRM-BIA 129. We moreover produced, in industrial conditions, an Emmental cheese using the same strain, in combination with Lactobacillus delbrueckii CNRZ327 and Streptococcus thermophilus LMD-9, as starters. Consumption of both cheeses was investigated with respect to prevention of Dextran Sodium Sulphate (DSS)-induced colitis in mice.
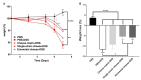
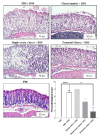
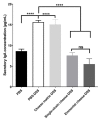
Results: Consumption of the single-strain experimental cheese, or of the industrial Emmental, both fermented by P. freudenreichii CIRM-BIA 129, reduced severity of subsequent DSS-induced colitis, weight loss, disease activity index and histological score. Both treatments, in a preventive way, reduced small bowel Immunoglobulin A (IgA) secretion, restored occludin gene expression and prevented induction of Tumor Necrosis Factor α (TNFα), Interferon γ (IFNγ) and Interleukin-17 (IL-17).
Conclusions: A combination of immunomodulatory strains of starter bacteria can be used to manufacture an anti-inflammatory cheese, as revealed in an animal model of colitis. This opens new perspectives for personalised nutrition in the context of IBD.
Keywords: Emmental; cheese; colitis; inflammation; inflammatory bowel disease; intestine; probiotic; propionibacteria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Combining selected immunomodulatory Propionibacterium freudenreichii and Lactobacillus delbrueckii strains: Reverse engineering development of an anti-inflammatory cheese.Mol Nutr Food Res. 2016 Apr;60(4):935-48. doi: 10.1002/mnfr.201500580. Epub 2015 Dec 29. Mol Nutr Food Res. 2016. PMID: 26640113
-
Propionibacterium freudenreichii CIRM-BIA 129 mitigates colitis through S layer protein B-dependent epithelial strengthening.Am J Physiol Gastrointest Liver Physiol. 2024 Feb 1;326(2):G163-G175. doi: 10.1152/ajpgi.00198.2023. Epub 2023 Nov 21. Am J Physiol Gastrointest Liver Physiol. 2024. PMID: 37988603
-
The Cheese Matrix Modulates the Immunomodulatory Properties of Propionibacterium freudenreichii CIRM-BIA 129 in Healthy Piglets.Front Microbiol. 2018 Oct 29;9:2584. doi: 10.3389/fmicb.2018.02584. eCollection 2018. Front Microbiol. 2018. PMID: 30420848 Free PMC article.
-
Improving the drying of Propionibacterium freudenreichii starter cultures.Appl Microbiol Biotechnol. 2021 May;105(9):3485-3494. doi: 10.1007/s00253-021-11273-3. Epub 2021 Apr 22. Appl Microbiol Biotechnol. 2021. PMID: 33885925 Review.
-
Dairy starters and fermented dairy products modulate gut mucosal immunity.Immunol Lett. 2022 Dec;251-252:91-102. doi: 10.1016/j.imlet.2022.11.002. Epub 2022 Nov 2. Immunol Lett. 2022. PMID: 36334759 Review.
Cited by
-
Modified Pulsatillae decoction inhibits DSS-induced ulcerative colitis in vitro and in vivo via IL-6/STAT3 pathway.BMC Complement Med Ther. 2020 Jun 9;20(1):179. doi: 10.1186/s12906-020-02974-9. BMC Complement Med Ther. 2020. PMID: 32517784 Free PMC article.
-
Dietary content and eating behavior in ulcerative colitis: a narrative review and future perspective.Nutr J. 2025 Jan 23;24(1):12. doi: 10.1186/s12937-025-01075-y. Nutr J. 2025. PMID: 39849464 Free PMC article. Review.
-
Therapeutic Effects of Probiotic Minas Frescal Cheese on the Attenuation of Ulcerative Colitis in a Murine Model.Front Microbiol. 2021 Mar 2;12:623920. doi: 10.3389/fmicb.2021.623920. eCollection 2021. Front Microbiol. 2021. PMID: 33737918 Free PMC article.
-
Strategies of Macrophages to Maintain Bone Homeostasis and Promote Bone Repair: A Narrative Review.J Funct Biomater. 2022 Dec 29;14(1):18. doi: 10.3390/jfb14010018. J Funct Biomater. 2022. PMID: 36662065 Free PMC article. Review.
-
Regulation of Yujin Powder alcoholic extracts on ILC3s-TD IgA-colonic mucosal flora axis of DSS-induced ulcerative colitis.Front Microbiol. 2022 Oct 20;13:1039884. doi: 10.3389/fmicb.2022.1039884. eCollection 2022. Front Microbiol. 2022. PMID: 36338041 Free PMC article.
References
-
- Diplock A.T., Aggett P., Ashwell M., Bornet F.R.J.B., Fern E.B., Roberfroid M. Scientific concepts of functional foods in europe: Consensus document. Br. J. Nutr. 1999;81:S1–S27. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

