Development of a Laboratory-safe and Low-cost Detection Protocol for SARS-CoV-2 of the Coronavirus Disease 2019 (COVID-19)
- PMID: 32156101
- PMCID: PMC7237269
- DOI: 10.5607/en20009
Development of a Laboratory-safe and Low-cost Detection Protocol for SARS-CoV-2 of the Coronavirus Disease 2019 (COVID-19)
Abstract
The severe acute respiratory coronavirus 2 (SARS-CoV-2), which emerged in December 2019 in Wuhan, China, has spread rapidly to over a dozen countries. Especially, the spike of case numbers in South Korea sparks pandemic worries. This virus is reported to spread mainly through person-to-person contact via respiratory droplets generated by coughing and sneezing, or possibly through surface contaminated by people coughing or sneezing on them. More critically, there have been reports about the possibility of this virus to transmit even before a virus-carrying person to show symptoms. Therefore, a low-cost, easy-access protocol for early detection of this virus is desperately needed. Here, we have established a real-time reverse-transcription PCR (rtPCR)-based assay protocol composed of easy specimen self-collection from a subject via pharyngeal swab, Trizol-based RNA purification, and SYBR Green-based rtPCR. This protocol shows an accuracy and sensitivity limit of 1-10 virus particles as we tested with a known lentivirus. The cost for each sample is estimated to be less than 15 US dollars. Overall time it takes for an entire protocol is estimated to be less than 4 hours. We propose a cost-effective, quick-and-easy method for early detection of SARS-CoV-2 at any conventional Biosafety Level II laboratories that are equipped with a rtPCR machine. Our newly developed protocol should be helpful for a first-hand screening of the asymptomatic virus-carriers for further prevention of transmission and early intervention and treatment for the rapidly propagating virus.
Keywords: COVID-19; Communicable diseases; Coronavirus; Diagnostic techniques and procedures; Emerging; Infectious disease; SARS virus.
Figures


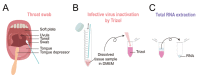




References
-
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. doi: 10.1001/jama.2020.1585. - DOI - PMC - PubMed
-
- Centers for Disease Control and Prevention. Centers for Disease Control; Atlanta: 2020. How COVID-19 spreads [Internet] Available from: https://www.cdc.gov/coronavirus/2019-ncov/about/transmission.html.
-
- Bendix A. Business Insider; New York: 2020. A person can carry and transmit COVID-19 without showing symptoms, scientists confirm [Internet] Available from: https://www.sciencealert.com/researchers-confirmed-patients-can-transmit....
-
- Hoehl S, Berger A, Kortenbusch M, Cinatl J, Bojkova D, Rabenau H, Behrens P, Böddinghaus B, Götsch U, Naujoks F, Neumann P, Schork J, Tiarks-Jungk P, Walczok A, Eickmann M, Vehreschild MJGT, Kann G, Wolf T, Gottschalk R, Ciesek S. Evidence of SARS-CoV-2 infection in returning travelers from Wuhan, China. N Engl J Med. 2020 doi: 10.1056/NEJMc2001899. doi: 10.1056/NEJMc2001899. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

