Biosynthesis and biology of mammalian GPI-anchored proteins
- PMID: 32156170
- PMCID: PMC7125958
- DOI: 10.1098/rsob.190290
Biosynthesis and biology of mammalian GPI-anchored proteins
Abstract
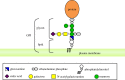
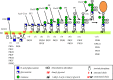
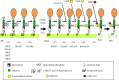
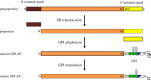
At least 150 human proteins are glycosylphosphatidylinositol-anchored proteins (GPI-APs). The protein moiety of GPI-APs lacking transmembrane domains is anchored to the plasma membrane with GPI covalently attached to the C-terminus. The GPI consists of the conserved core glycan, phosphatidylinositol and glycan side chains. The entire GPI-AP is anchored to the outer leaflet of the lipid bilayer by insertion of fatty chains of phosphatidylinositol. Because of GPI-dependent membrane anchoring, GPI-APs have some unique characteristics. The most prominent feature of GPI-APs is their association with membrane microdomains or membrane rafts. In the polarized cells such as epithelial cells, many GPI-APs are exclusively expressed in the apical surfaces, whereas some GPI-APs are preferentially expressed in the basolateral surfaces. Several GPI-APs act as transcytotic transporters carrying their ligands from one compartment to another. Some GPI-APs are shed from the membrane after cleavage within the GPI by a GPI-specific phospholipase or a glycosidase. In this review, I will summarize the current understanding of GPI-AP biosynthesis in mammalian cells and discuss examples of GPI-dependent functions of mammalian GPI-APs.
Keywords: GPI deficiency; biosynthetic pathway; glycosylphosphatidylinositol; post-translational modification; protein shedding.
Conflict of interest statement
I declare I have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
