Evaluation of a quantitative RT-PCR assay for the detection of the emerging coronavirus SARS-CoV-2 using a high throughput system
- PMID: 32156329
- PMCID: PMC7068162
- DOI: 10.2807/1560-7917.ES.2020.25.9.2000152
Evaluation of a quantitative RT-PCR assay for the detection of the emerging coronavirus SARS-CoV-2 using a high throughput system
Erratum in
-
Authors' correction for Euro Surveill. 2020;25(9).Euro Surveill. 2020 Mar;25(10):20200312c. doi: 10.2807/1560-7917.ES.2020.25.10.20200312c. Euro Surveill. 2020. PMID: 32183936 Free PMC article. No abstract available.
Abstract
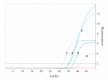
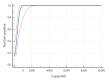
Facing the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), high-volume respiratory testing is demanded in laboratories worldwide. We evaluated the performance of a molecular assay for the detection of SARS-CoV-2 on a high-throughput platform, the cobas 6800, using the 'open channel' for integration of a laboratory-developed assay. We observed good analytical performance in clinical specimens. The fully automated workflow enables high-throughput testing with minimal hands-on time, while offering fast and reliable results.
Keywords: COVID-19; SARS-CoV-2; automation; high-throughput PCR; molecular diagnostic.
Conflict of interest statement
Figures
Comment on
-
Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR.Euro Surveill. 2020 Jan;25(3):2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. Euro Surveill. 2020. PMID: 31992387 Free PMC article.
References
-
- Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, et al. Severe acute respiratory syndrome-related coronavirus: The species and its viruses – a statement of the Coronavirus Study Group. bioRxiv. 2020;937862: (Preprint). 10.1101/2020.02.07.937862 - DOI
-
- Centers for Disease Control and Prevention (CDC). Research use only 2019-novel coronavirus (2019-nCoV) real-time rt-pcr primer and probe information. Atlanta: CDC; 2020.Available from: https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-panel-primer-probes...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous