Rapid establishment of laboratory diagnostics for the novel coronavirus SARS-CoV-2 in Bavaria, Germany, February 2020
- PMID: 32156330
- PMCID: PMC7068163
- DOI: 10.2807/1560-7917.ES.2020.25.9.2000173
Rapid establishment of laboratory diagnostics for the novel coronavirus SARS-CoV-2 in Bavaria, Germany, February 2020
Abstract
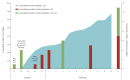
The need for timely establishment of diagnostic assays arose when Germany was confronted with the first travel-associated outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Europe. We describe our laboratory experiences during a large contact tracing investigation, comparing previously published real-time RT-PCR assays in different PCR systems and a commercial kit. We found that assay performance using the same primers and probes with different PCR systems varied and the commercial kit performed well.
Keywords: COVID-19; Corona Virus; Infectious disease; Laboratory Diagnostics; Molecular Testing; Real-Time PCR; SARS-CoV-2; outbreaks.
Conflict of interest statement
Figures


References
-
- World Health Organization (WHO). Disease outbreak news: novel coronavirus—Thailand (ex-China). Geneva; WHO;14 Jan 2020. Available from; https://www.who.int/csr/don/14-january-2020-novel-coronavirus-thailand-e...
-
- World Health Organization (WHO). Disease outbreak news: novel coronavirus—Japan (ex-China). Geneva; WHO;17 Jan 2020. Available from; https://www.who.int/csr/don/17-january-2020-novel-coronavirus-japan-ex-c...
-
- World Health Organization (WHO). Disease outbreak news: novel coronavirus—Republic of Korea (ex-China). Geneva; WHO; 21 Jan 2020. Available from; https://www.who.int/csr/don/21-january-2020-novel-coronavirus-republic-o...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
