Arrestin domain-containing 3 (Arrdc3) modulates insulin action and glucose metabolism in liver
- PMID: 32156724
- PMCID: PMC7104271
- DOI: 10.1073/pnas.1922370117
Arrestin domain-containing 3 (Arrdc3) modulates insulin action and glucose metabolism in liver
Abstract
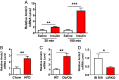
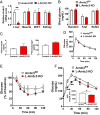
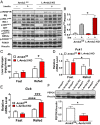
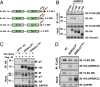
Insulin action in the liver is critical for glucose homeostasis through regulation of glycogen synthesis and glucose output. Arrestin domain-containing 3 (Arrdc3) is a member of the α-arrestin family previously linked to human obesity. Here, we show that Arrdc3 is differentially regulated by insulin in vivo in mice undergoing euglycemic-hyperinsulinemic clamps, being highly up-regulated in liver and down-regulated in muscle and fat. Mice with liver-specific knockout (KO) of the insulin receptor (IR) have a 50% reduction in Arrdc3 messenger RNA, while, conversely, mice with liver-specific KO of Arrdc3 (L-Arrdc3 KO) have increased IR protein in plasma membrane. This leads to increased hepatic insulin sensitivity with increased phosphorylation of FOXO1, reduced expression of PEPCK, and increased glucokinase expression resulting in reduced hepatic glucose production and increased hepatic glycogen accumulation. These effects are due to interaction of ARRDC3 with IR resulting in phosphorylation of ARRDC3 on a conserved tyrosine (Y382) in the carboxyl-terminal domain. Thus, Arrdc3 is an insulin target gene, and ARRDC3 protein directly interacts with IR to serve as a feedback regulator of insulin action in control of liver metabolism.
Keywords: Arrdc3; alpha arrestins; glucose metabolism; insulin action; liver.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Saltiel A. R., Kahn C. R., Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414, 799–806 (2001). - PubMed
-
- Schmoll D., et al. , Regulation of glucose-6-phosphatase gene expression by protein kinase Balpha and the forkhead transcription factor FKHR. Evidence for insulin response unit-dependent and -independent effects of insulin on promoter activity. J. Biol. Chem. 275, 36324–36333 (2000). - PubMed
-
- Biddinger S. B., Kahn C. R., From mice to men: Insights into the insulin resistance syndromes. Annu. Rev. Physiol. 68, 123–158 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

