Potential for release of pulmonary toxic ketene from vaping pyrolysis of vitamin E acetate
- PMID: 32156732
- PMCID: PMC7104367
- DOI: 10.1073/pnas.1920925117
Potential for release of pulmonary toxic ketene from vaping pyrolysis of vitamin E acetate
Abstract
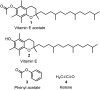
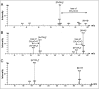
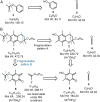
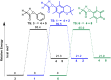
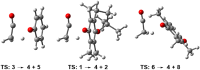
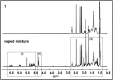
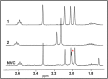
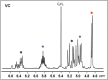
A combined analytical, theoretical, and experimental study has shown that the vaping of vitamin E acetate has the potential to produce exceptionally toxic ketene gas, which may be a contributing factor to the upsurge in pulmonary injuries associated with using e-cigarette/vaping products. Additionally, the pyrolysis of vitamin E acetate also produces carcinogen alkenes and benzene for which the negative long-term medical effects are well recognized. As temperatures reached in vaping devices can be equivalent to a laboratory pyrolysis apparatus, the potential for unexpected chemistries to take place on individual components within a vape mixture is high. Educational programs to inform of the danger are now required, as public perception has grown that vaping is not harmful.
Keywords: ketene; lung injury; pyrolysis; vaping; vitamin E acetate.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures
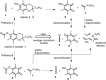
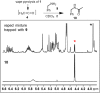

Comment in
-
Toxic ketene gas forms on vaping vitamin E acetate prompting interest in its possible role in the EVALI outbreak.Proc Natl Acad Sci U S A. 2020 Apr 7;117(14):7553-7554. doi: 10.1073/pnas.2003384117. Epub 2020 Mar 31. Proc Natl Acad Sci U S A. 2020. PMID: 32234786 Free PMC article. No abstract available.
References
-
- Ledford H., Scientists chase cause of mysterious vaping illness as death toll rises. Nature 574, 303–304 (2019). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

