Targeting Glycosylated PD-1 Induces Potent Antitumor Immunity
- PMID: 32156778
- PMCID: PMC7272274
- DOI: 10.1158/0008-5472.CAN-19-3133
Targeting Glycosylated PD-1 Induces Potent Antitumor Immunity
Abstract
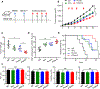
Immunotherapies targeting programmed cell death protein 1 (PD-1) and programmed cell death 1 ligand 1 (PD-L1) immune checkpoints represent a major breakthrough in cancer treatment. PD-1 is an inhibitory receptor expressed on the surface of activated T cells that dampens T-cell receptor (TCR)/CD28 signaling by engaging with its ligand PD-L1 expressed on cancer cells. Despite the clinical success of PD-1 blockade using mAbs, most patients do not respond to the treatment, and the underlying regulatory mechanisms of PD-1 remain incompletely defined. Here we show that PD-1 is extensively N-glycosylated in T cells and the intensities of its specific glycoforms are altered upon TCR activation. Glycosylation was critical for maintaining PD-1 protein stability and cell surface localization. Glycosylation of PD-1, especially at the N58 site, was essential for mediating its interaction with PD-L1. The mAb STM418 specifically targeted glycosylated PD-1, exhibiting higher binding affinity to PD-1 than FDA-approved PD-1 antibodies, potently inhibiting PD-L1/PD-1 binding, and enhancing antitumor immunity. Together, these findings provide novel insights into the functional significance of PD-1 glycosylation and offer a rationale for targeting glycosylated PD-1 as a potential strategy for immunotherapy. SIGNIFICANCE: These findings demonstrate that glycosylation of PD-1 is functionally significant and targeting glycosylated PD-1 may serve as a means to improve immunotherapy response.
©2020 American Association for Cancer Research.
Conflict of interest statement
Figures
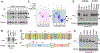





References
-
- Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol 2007;19:813–24 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

