CO2/HCO3- Accelerates Iron Reduction through Phenolic Compounds
- PMID: 32156807
- PMCID: PMC7064749
- DOI: 10.1128/mBio.00085-20
CO2/HCO3- Accelerates Iron Reduction through Phenolic Compounds
Abstract
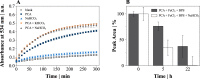
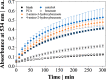
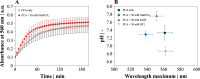
Iron is a vital mineral for almost all living organisms and has a pivotal role in central metabolism. Despite its great abundance on earth, the accessibility for microorganisms is often limited, because poorly soluble ferric iron (Fe3+) is the predominant oxidation state in an aerobic environment. Hence, the reduction of Fe3+ is of essential importance to meet the cellular demand of ferrous iron (Fe2+) but might become detrimental as excessive amounts of intracellular Fe2+ tend to undergo the cytotoxic Fenton reaction in the presence of hydrogen peroxide. We demonstrate that the complex formation rate of Fe3+ and phenolic compounds like protocatechuic acid was increased by 46% in the presence of HCO3- and thus accelerated the subsequent redox reaction, yielding reduced Fe2+ Consequently, elevated CO2/HCO3- levels increased the intracellular Fe2+ availability, which resulted in at least 50% higher biomass-specific fluorescence of a DtxR-based Corynebacterium glutamicum reporter strain, and stimulated growth. Since the increased Fe2+ availability was attributed to the interaction of HCO3- and chemical iron reduction, the abiotic effect postulated in this study is of general relevance in geochemical and biological environments.IMPORTANCE In an oxygenic environment, poorly soluble Fe3+ must be reduced to meet the cellular Fe2+ demand. This study demonstrates that elevated CO2/HCO3- levels accelerate chemical Fe3+ reduction through phenolic compounds, thus increasing intracellular Fe2+ availability. A number of biological environments are characterized by the presence of phenolic compounds and elevated HCO3- levels and include soil habitats and the human body. Fe2+ availability is of particular interest in the latter, as it controls the infectiousness of pathogens. Since the effect postulated here is abiotic, it generally affects the Fe2+ distribution in nature.
Keywords: Corynebacterium glutamicum; DtxR; bicarbonate; carbon dioxide; iron homeostasis; iron reduction; pathogens.
Copyright © 2020 Müller et al.
Figures


Similar articles
-
Impact of CO2/HCO3- Availability on Anaplerotic Flux in Pyruvate Dehydrogenase Complex-Deficient Corynebacterium glutamicum Strains.J Bacteriol. 2019 Sep 20;201(20):e00387-19. doi: 10.1128/JB.00387-19. Print 2019 Oct 15. J Bacteriol. 2019. PMID: 31358612 Free PMC article.
-
Impact of different CO2/HCO3- levels on metabolism and regulation in Corynebacterium glutamicum.J Biotechnol. 2013 Dec;168(4):331-40. doi: 10.1016/j.jbiotec.2013.10.005. Epub 2013 Oct 16. J Biotechnol. 2013. PMID: 24140290
-
Iron oxidation stimulates organic matter decomposition in humid tropical forest soils.Glob Chang Biol. 2013 Sep;19(9):2804-13. doi: 10.1111/gcb.12229. Epub 2013 Jul 14. Glob Chang Biol. 2013. PMID: 23606589
-
Avoiding high-valent iron intermediates: superoxide reductase and rubrerythrin.J Inorg Biochem. 2006 Apr;100(4):679-93. doi: 10.1016/j.jinorgbio.2005.12.017. Epub 2006 Feb 28. J Inorg Biochem. 2006. PMID: 16504301 Review.
-
Bioenergetic challenges of microbial iron metabolisms.Trends Microbiol. 2011 Jul;19(7):330-40. doi: 10.1016/j.tim.2011.05.001. Epub 2011 Jun 12. Trends Microbiol. 2011. PMID: 21664821 Review.
Cited by
-
Using ferrous-oxidizing bacteria to enhance the performance of a pH neutral all-iron flow battery.iScience. 2023 Nov 29;27(1):108595. doi: 10.1016/j.isci.2023.108595. eCollection 2024 Jan 19. iScience. 2023. PMID: 38174320 Free PMC article.
-
Response surface-based media optimization for astaxanthin production in Corynebacterium glutamicum.Front Bioeng Biotechnol. 2025 Mar 11;13:1516522. doi: 10.3389/fbioe.2025.1516522. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40134774 Free PMC article.
-
Antioxidant and Antibacterial Activities of the Leaf and Stem Extracts of Combretum molle (R. Br. ex G. Don.) Engl. & Diels.Plants (Basel). 2023 Apr 25;12(9):1757. doi: 10.3390/plants12091757. Plants (Basel). 2023. PMID: 37176814 Free PMC article.
-
A Timed Off-Switch for Dynamic Control of Gene Expression in Corynebacterium Glutamicum.Front Bioeng Biotechnol. 2021 Jul 29;9:704681. doi: 10.3389/fbioe.2021.704681. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34395409 Free PMC article.
-
Metabolic engineering of Corynebacterium glutamicum for fatty alcohol production from glucose and wheat straw hydrolysate.Biotechnol Biofuels Bioprod. 2023 Jul 18;16(1):116. doi: 10.1186/s13068-023-02367-3. Biotechnol Biofuels Bioprod. 2023. PMID: 37464396 Free PMC article.
References
-
- Proulx-Curry PM, Chasteen ND. 1995. Molecular aspects of iron uptake and storage in ferritin. Coord Chem Rev 144:347–368. doi:10.1016/0010-8545(95)01148-I. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

