Cancer associated fibroblast FAK regulates malignant cell metabolism
- PMID: 32157087
- PMCID: PMC7064590
- DOI: 10.1038/s41467-020-15104-3
Cancer associated fibroblast FAK regulates malignant cell metabolism
Abstract
Emerging evidence suggests that cancer cell metabolism can be regulated by cancer-associated fibroblasts (CAFs), but the mechanisms are poorly defined. Here we show that CAFs regulate malignant cell metabolism through pathways under the control of FAK. In breast and pancreatic cancer patients we find that low FAK expression, specifically in the stromal compartment, predicts reduced overall survival. In mice, depletion of FAK in a subpopulation of CAFs regulates paracrine signals that increase malignant cell glycolysis and tumour growth. Proteomic and phosphoproteomic analysis in our mouse model identifies metabolic alterations which are reflected at the transcriptomic level in patients with low stromal FAK. Mechanistically we demonstrate that FAK-depletion in CAFs increases chemokine production, which via CCR1/CCR2 on cancer cells, activate protein kinase A, leading to enhanced malignant cell glycolysis. Our data uncover mechanisms whereby stromal fibroblasts regulate cancer cell metabolism independent of genetic mutations in cancer cells.
Conflict of interest statement
The authors have no competing interests except Y.W., J.J.C., R.S., P.Z. and T.J.S. who are all employees of ChemoCentryx and developed and provided the CCr1i/CCR2i double inhibitor for the study.
Figures


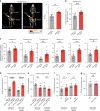

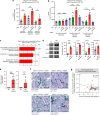
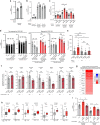
References
Publication types
MeSH terms
Substances
Grants and funding
- MC_UU_12022/6/MRC_/Medical Research Council/United Kingdom
- C8218/A21453/CRUK_/Cancer Research UK/United Kingdom
- DTP 2013/MRC_/Medical Research Council/United Kingdom
- C16420/A18066/CRUK_/Cancer Research UK/United Kingdom
- A19771/CRUK_/Cancer Research UK/United Kingdom
- C8218/A18673/CRUK_/Cancer Research UK/United Kingdom
- A16354/CRUK_/Cancer Research UK/United Kingdom
- 19771/CRUK_/Cancer Research UK/United Kingdom
- 202778/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- BB/M006174/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- A14895/CRUK_/Cancer Research UK/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

