Nanohybrids of a MXene and transition metal dichalcogenide for selective detection of volatile organic compounds
- PMID: 32157089
- PMCID: PMC7064528
- DOI: 10.1038/s41467-020-15092-4
Nanohybrids of a MXene and transition metal dichalcogenide for selective detection of volatile organic compounds
Abstract
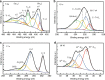
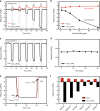
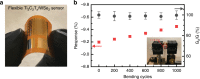
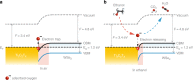
Two-dimensional transition metal carbides/nitrides, known as MXenes, have been recently receiving attention for gas sensing. However, studies on hybridization of MXenes and 2D transition metal dichalcogenides as gas-sensing materials are relatively rare at this time. Herein, Ti3C2Tx and WSe2 are selected as model materials for hybridization and implemented toward detection of various volatile organic compounds. The Ti3C2Tx/WSe2 hybrid sensor exhibits low noise level, ultrafast response/recovery times, and good flexibility for various volatile organic compounds. The sensitivity of the hybrid sensor to ethanol is improved by over 12-fold in comparison with pristine Ti3C2Tx. Moreover, the hybridization process provides an effective strategy against MXene oxidation by restricting the interaction of water molecules from the edges of Ti3C2Tx. An enhancement mechanism for Ti3C2Tx/WSe2 heterostructured materials is proposed for highly sensitive and selective detection of oxygen-containing volatile organic compounds. The scientific findings of this work could guide future exploration of next-generation field-deployable sensors.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Surface Functionalization of Ti3C2Tx MXene with Highly Reliable Superhydrophobic Protection for Volatile Organic Compounds Sensing.ACS Nano. 2020 Sep 22;14(9):11490-11501. doi: 10.1021/acsnano.0c03896. Epub 2020 Sep 2. ACS Nano. 2020. PMID: 32857499
-
Research status of gas sensing performance of Ti3C2Tx-based gas sensors: A mini review.Front Chem. 2022 Oct 3;10:1037732. doi: 10.3389/fchem.2022.1037732. eCollection 2022. Front Chem. 2022. PMID: 36262339 Free PMC article. Review.
-
Highly Selective H2S Gas Sensor Based on Ti3C2Tx MXene-Organic Composites.ACS Appl Mater Interfaces. 2023 Feb 8;15(5):7063-7073. doi: 10.1021/acsami.2c19883. Epub 2023 Jan 24. ACS Appl Mater Interfaces. 2023. PMID: 36694305 Free PMC article.
-
Unlocking the Potential of Ti3C2Tx MXene: Present Trends and Future Developments of Gas Sensing.Micromachines (Basel). 2025 Jan 29;16(2):159. doi: 10.3390/mi16020159. Micromachines (Basel). 2025. PMID: 40047593 Free PMC article. Review.
-
Room-temperature chemiresistive ammonia sensors based on 2D MXenes and their hybrids: recent developments and future prospects.Dalton Trans. 2023 Oct 10;52(39):13831-13851. doi: 10.1039/d3dt02401f. Dalton Trans. 2023. PMID: 37724340 Review.
Cited by
-
Application Prospects of MXenes Materials Modifications for Sensors.Micromachines (Basel). 2023 Jan 18;14(2):247. doi: 10.3390/mi14020247. Micromachines (Basel). 2023. PMID: 36837947 Free PMC article. Review.
-
MXenes as Emerging Materials: Synthesis, Properties, and Applications.Molecules. 2022 Aug 1;27(15):4909. doi: 10.3390/molecules27154909. Molecules. 2022. PMID: 35956859 Free PMC article. Review.
-
Enhanced Sensitivity in Photovoltaic 2D MoS2/Te Heterojunction VOC Sensors.Small. 2024 Dec;20(49):e2402464. doi: 10.1002/smll.202402464. Epub 2024 Jul 26. Small. 2024. PMID: 39058241 Free PMC article.
-
A comprehensive overview of recent progress in MXene-based polymer composites: Their fabrication processes, advanced applications, and prospects.Heliyon. 2024 Aug 30;10(17):e37030. doi: 10.1016/j.heliyon.2024.e37030. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39319124 Free PMC article. Review.
-
VOCs Sensing by Metal Oxides, Conductive Polymers, and Carbon-Based Materials.Nanomaterials (Basel). 2021 Feb 22;11(2):552. doi: 10.3390/nano11020552. Nanomaterials (Basel). 2021. PMID: 33671783 Free PMC article. Review.
References
-
- Swan M. Sensor mania! the Internet of Things, wearable computing, objective metrics, and the quantified self 2.0. J. Sens. Actuator Netw. 2012;1:217–253. doi: 10.3390/jsan1030217. - DOI
-
- Peng J, Chen X, Ong WJ, Zhao X, Li N. Surface and heterointerface engineering of 2D MXenes and their nanocomposites: insights into electro- and photocatalysis. Chem. 2019;5:18–50. doi: 10.1016/j.chempr.2018.08.037. - DOI
LinkOut - more resources
Full Text Sources

