The temporal structure of the inner retina at a single glance
- PMID: 32157103
- PMCID: PMC7064538
- DOI: 10.1038/s41598-020-60214-z
The temporal structure of the inner retina at a single glance
Abstract
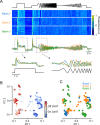
The retina decomposes visual stimuli into parallel channels that encode different features of the visual environment. Central to this computation is the synaptic processing in a dense layer of neuropil, the so-called inner plexiform layer (IPL). Here, different types of bipolar cells stratifying at distinct depths relay the excitatory feedforward drive from photoreceptors to amacrine and ganglion cells. Current experimental techniques for studying processing in the IPL do not allow imaging the entire IPL simultaneously in the intact tissue. Here, we extend a two-photon microscope with an electrically tunable lens allowing us to obtain optical vertical slices of the IPL, which provide a complete picture of the response diversity of bipolar cells at a "single glance". The nature of these axial recordings additionally allowed us to isolate and investigate batch effects, i.e. inter-experimental variations resulting in systematic differences in response speed. As a proof of principle, we developed a simple model that disentangles biological from experimental causes of variability and allowed us to recover the characteristic gradient of response speeds across the IPL with higher precision than before. Our new framework will make it possible to study the computations performed in the central synaptic layer of the retina more efficiently.
Conflict of interest statement
The authors declare no competing interests.
Figures







Similar articles
-
Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses.J Comp Neurol. 2003 Jun 2;460(3):380-93. doi: 10.1002/cne.10652. J Comp Neurol. 2003. PMID: 12692856
-
Disruption of transient photoreceptor targeting within the inner plexiform layer following early ablation of cholinergic amacrine cells in the ferret.Vis Neurosci. 2001 Sep-Oct;18(5):741-51. doi: 10.1017/s095252380118507x. Vis Neurosci. 2001. PMID: 11925009
-
Organization of the inner plexiform layer of the turtle retina: an electron microscopic study.J Comp Neurol. 1988 Jun 8;272(2):280-92. doi: 10.1002/cne.902720210. J Comp Neurol. 1988. PMID: 3397409
-
General features of inhibition in the inner retina.J Physiol. 2017 Aug 15;595(16):5507-5515. doi: 10.1113/JP273648. Epub 2017 May 4. J Physiol. 2017. PMID: 28332227 Free PMC article. Review.
-
Inhibitory Interneurons in the Retina: Types, Circuitry, and Function.Annu Rev Vis Sci. 2017 Sep 15;3:1-24. doi: 10.1146/annurev-vision-102016-061345. Epub 2017 Jun 15. Annu Rev Vis Sci. 2017. PMID: 28617659 Review.
Cited by
-
Distributed feature representations of natural stimuli across parallel retinal pathways.Nat Commun. 2024 Mar 1;15(1):1920. doi: 10.1038/s41467-024-46348-y. Nat Commun. 2024. PMID: 38429280 Free PMC article.
-
Most discriminative stimuli for functional cell type clustering.ArXiv [Preprint]. 2024 Mar 14:arXiv:2401.05342v2. ArXiv. 2024. PMID: 38560735 Free PMC article. Preprint.
-
Center-surround interactions underlie bipolar cell motion sensitivity in the mouse retina.Nat Commun. 2022 Sep 26;13(1):5574. doi: 10.1038/s41467-022-32762-7. Nat Commun. 2022. PMID: 36163124 Free PMC article.
-
A chromatic feature detector in the retina signals visual context changes.Elife. 2024 Oct 4;13:e86860. doi: 10.7554/eLife.86860. Elife. 2024. PMID: 39365730 Free PMC article.
-
Temperature and species-dependent regulation of browning in retrobulbar fat.Sci Rep. 2021 Feb 4;11(1):3094. doi: 10.1038/s41598-021-82672-9. Sci Rep. 2021. PMID: 33542375 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

