Downregulation of respiratory complex I mediates major signalling changes triggered by TOR activation
- PMID: 32157127
- PMCID: PMC7064613
- DOI: 10.1038/s41598-020-61244-3
Downregulation of respiratory complex I mediates major signalling changes triggered by TOR activation
Abstract
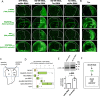
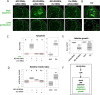
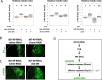
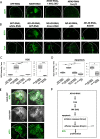
Mitochondrial dysfunctions belong amongst the most common metabolic diseases but the signalling networks that lead to the manifestation of a disease phenotype are often not well understood. We identified the subunits of respiratory complex I, III and IV as mediators of major signalling changes during Drosophila wing disc development. Their downregulation in larval wing disc leads to robust stimulation of TOR activity, which in turn orchestrates a complex downstream signalling network. Specifically, after downregulation of the complex I subunit ND-49 (mammalian NDUFS2), TOR activates JNK to induce cell death and ROS production essential for the stimulation of compensatory apoptosis-induced proliferation within the tissue. Additionally, TOR upregulates Notch and JAK/STAT signalling and it directs glycolytic switch of the target tissue. Our results highlight the central role of TOR signalling in mediating the complex response to mitochondrial respiratory dysfunction and they provide a rationale why the disease symptoms associated with respiratory dysfunctions are often alleviated by mTOR inhibitors.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Zika virus non-structural protein NS4A restricts eye growth in Drosophila through regulation of JAK/STAT signaling.Dis Model Mech. 2020 Apr 30;13(4):dmm040816. doi: 10.1242/dmm.040816. Dis Model Mech. 2020. PMID: 32152180 Free PMC article.
-
A positive role of Sin3A in regulating Notch signaling during Drosophila wing development.Cell Signal. 2019 Jan;53:184-189. doi: 10.1016/j.cellsig.2018.10.008. Epub 2018 Oct 12. Cell Signal. 2019. PMID: 30316814
-
Localised JAK/STAT pathway activation is required for Drosophila wing hinge development.PLoS One. 2013 May 31;8(5):e65076. doi: 10.1371/journal.pone.0065076. Print 2013. PLoS One. 2013. PMID: 23741461 Free PMC article.
-
The JAK/STAT pathway and Drosophila development.Bioessays. 2001 Dec;23(12):1138-47. doi: 10.1002/bies.10016. Bioessays. 2001. PMID: 11746233 Review.
-
JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions.Development. 2006 Jul;133(14):2605-16. doi: 10.1242/dev.02411. Development. 2006. PMID: 16794031 Review.
Cited by
-
The mitochondrial ribosomal protein mRpL4 regulates Notch signaling.EMBO Rep. 2023 Jun 5;24(6):e55764. doi: 10.15252/embr.202255764. Epub 2023 Apr 3. EMBO Rep. 2023. PMID: 37009823 Free PMC article.
-
Hyperpolarized mitochondria accumulate in Drosophila Hipk-overexpressing cells to drive tumor-like growth.J Cell Sci. 2020 Dec 9;133(23):jcs250944. doi: 10.1242/jcs.250944. J Cell Sci. 2020. PMID: 33199523 Free PMC article.
-
The ER protein Creld regulates ER-mitochondria contact dynamics and respiratory complex 1 activity.Sci Adv. 2022 Jul 22;8(29):eabo0155. doi: 10.1126/sciadv.abo0155. Epub 2022 Jul 22. Sci Adv. 2022. PMID: 35867795 Free PMC article.
-
Insulin and IGF-1 receptors regulate complex I-dependent mitochondrial bioenergetics and supercomplexes via FoxOs in muscle.J Clin Invest. 2021 Sep 15;131(18):e146415. doi: 10.1172/JCI146415. J Clin Invest. 2021. PMID: 34343133 Free PMC article.
-
Reduced expression of mitochondrial complex I subunit Ndufs2 does not impact healthspan in mice.Sci Rep. 2022 Mar 25;12(1):5196. doi: 10.1038/s41598-022-09074-3. Sci Rep. 2022. PMID: 35338200 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

