Long-range replica exchange molecular dynamics guided drug repurposing against tyrosine kinase PtkA of Mycobacterium tuberculosis
- PMID: 32157138
- PMCID: PMC7064604
- DOI: 10.1038/s41598-020-61132-w
Long-range replica exchange molecular dynamics guided drug repurposing against tyrosine kinase PtkA of Mycobacterium tuberculosis
Abstract
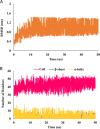
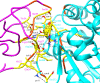
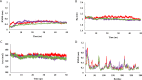
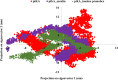
Tuberculosis (TB) is a leading cause of death worldwide and its impact has intensified due to the emergence of multi drug-resistant (MDR) and extensively drug-resistant (XDR) TB strains. Protein phosphorylation plays a vital role in the virulence of Mycobacterium tuberculosis (M.tb) mediated by protein kinases. Protein tyrosine phosphatase A (MptpA) undergoes phosphorylation by a unique tyrosine-specific kinase, protein tyrosine kinase A (PtkA), identified in the M.tb genome. PtkA phosphorylates PtpA on the tyrosine residues at positions 128 and 129, thereby increasing PtpA activity and promoting pathogenicity of MptpA. In the present study, we performed an extensive investigation of the conformational behavior of the intrinsically disordered domain (IDD) of PtkA using replica exchange molecular dynamics simulations. Long-term molecular dynamics (MD) simulations were performed to elucidate the role of IDD on the catalytic activity of kinase core domain (KCD) of PtkA. This was followed by identification of the probable inhibitors of PtkA using drug repurposing to block the PtpA-PtkA interaction. The inhibitory role of IDD on KCD has already been established; however, various analyses conducted in the present study showed that IDDPtkA had a greater inhibitory effect on the catalytic activity of KCDPtkA in the presence of the drugs esculin and inosine pranobex. The binding of drugs to PtkA resulted in formation of stable complexes, indicating that these two drugs are potentially useful as inhibitors of M.tb.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
The domain architecture of PtkA, the first tyrosine kinase from Mycobacterium tuberculosis, differs from the conventional kinase architecture.J Biol Chem. 2018 Jul 27;293(30):11823-11836. doi: 10.1074/jbc.RA117.000120. Epub 2018 Jun 8. J Biol Chem. 2018. PMID: 29884774 Free PMC article.
-
Structural characterization of the intrinsically disordered domain of Mycobacterium tuberculosis protein tyrosine kinase A.FEBS Lett. 2018 Apr;592(7):1233-1245. doi: 10.1002/1873-3468.13022. Epub 2018 Mar 25. FEBS Lett. 2018. PMID: 29494752
-
Phosphorylation of Mycobacterium tuberculosis protein tyrosine kinase A PtkA by Ser/Thr protein kinases.Biochem Biophys Res Commun. 2015 Nov 13;467(2):421-6. doi: 10.1016/j.bbrc.2015.09.124. Epub 2015 Sep 26. Biochem Biophys Res Commun. 2015. PMID: 26417687
-
New strategies in fighting TB: targeting Mycobacterium tuberculosis-secreted phosphatases MptpA & MptpB.Future Med Chem. 2010 Aug;2(8):1325-37. doi: 10.4155/fmc.10.214. Future Med Chem. 2010. PMID: 21426021 Review.
-
Exploring the role of the protein tyrosine kinase a (PtkA) in mycobacterial intracellular survival.Tuberculosis (Edinb). 2023 Sep;142:102398. doi: 10.1016/j.tube.2023.102398. Epub 2023 Aug 24. Tuberculosis (Edinb). 2023. PMID: 37657276 Review.
Cited by
-
Ligand-induced shifts in conformational ensembles that describe transcriptional activation.Elife. 2022 Oct 12;11:e80140. doi: 10.7554/eLife.80140. Elife. 2022. PMID: 36222302 Free PMC article.
-
Enhancing tuberculosis vaccine development: a deconvolution neural network approach for multi-epitope prediction.Sci Rep. 2024 May 6;14(1):10375. doi: 10.1038/s41598-024-59291-1. Sci Rep. 2024. PMID: 38710737 Free PMC article.
-
From infection niche to therapeutic target: the intracellular lifestyle of Mycobacterium tuberculosis.Microbiology (Reading). 2021 Apr;167(4):001041. doi: 10.1099/mic.0.001041. Microbiology (Reading). 2021. PMID: 33826491 Free PMC article. Review.
-
An immunoinformatics approach to design a multi-epitope vaccine against Mycobacterium tuberculosis exploiting secreted exosome proteins.Sci Rep. 2021 Jul 5;11(1):13836. doi: 10.1038/s41598-021-93266-w. Sci Rep. 2021. PMID: 34226593 Free PMC article.
-
Atomistic Simulations of Functionalized Nano-Materials for Biosensors Applications.Int J Mol Sci. 2022 Jan 27;23(3):1484. doi: 10.3390/ijms23031484. Int J Mol Sci. 2022. PMID: 35163407 Free PMC article. Review.
References
-
- Pushpakom, S. et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov (2018). - PubMed
-
- Av-Gay, Y. a. D., V. In Tuberculosis and the Tubercle Bacillus (eds. Cole, S. T., Eisenach, K. D., McMurray, D. N. & Jacobs, W. R.) 359–367 (ASM Press Washington, 2005).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

