Arteriovenous Malformation MAP2K1 Mutation Causes Local Cartilage Overgrowth by a Cell-Non Autonomous Mechanism
- PMID: 32157142
- PMCID: PMC7064492
- DOI: 10.1038/s41598-020-61444-x
Arteriovenous Malformation MAP2K1 Mutation Causes Local Cartilage Overgrowth by a Cell-Non Autonomous Mechanism
Abstract
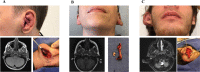
Extracranial arteriovenous malformation (AVM) is most commonly caused by MAP2K1 mutations in the endothelial cell. The purpose of this study was to determine if local tissue overgrowth associated with AVM is caused by direct or indirect effects of the MAP2K1 mutation (i.e., cell-autonomous or cell-non autonomous). Because cartilage does not have blood vessels, we studied ear AVMs to determine if overgrown cartilage contained AVM-causing mutations. Cartilage was separated from its surrounding tissue and isolated by laser capture microdissection. Droplet digital PCR (ddPCR) was used to identify MAP2K1 mutations. MAP2K1 (p.K57N) variants were present in the tissue adjacent to the cartilage [mutant allele frequency (MAF) 6-8%], and were enriched in endothelial cells (MAF 51%) compared to non-endothelial cells (MAF 0%). MAP2K1 mutations were not identified in the overgrown cartilage, and thus local cartilage overgrowth likely results from the effects of adjacent mutant blood vessels (i.e., cell-non autonomous).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Arteriovenous malformation Map2k1 mutation affects vasculogenesis.Sci Rep. 2023 Jul 8;13(1):11074. doi: 10.1038/s41598-023-35301-6. Sci Rep. 2023. PMID: 37422456 Free PMC article.
-
Somatic MAP2K1 Mutations Are Associated with Extracranial Arteriovenous Malformation.Am J Hum Genet. 2017 Mar 2;100(3):546-554. doi: 10.1016/j.ajhg.2017.01.018. Epub 2017 Feb 9. Am J Hum Genet. 2017. PMID: 28190454 Free PMC article.
-
Endothelial MAP2K1 mutations in arteriovenous malformation activate the RAS/MAPK pathway.Biochem Biophys Res Commun. 2020 Aug 20;529(2):450-454. doi: 10.1016/j.bbrc.2020.06.022. Epub 2020 Jul 1. Biochem Biophys Res Commun. 2020. PMID: 32703450 Free PMC article.
-
[Neonatal capillary malformation-arteriovenous malformation complicated with acute heart failure: a case report and literature review].Zhonghua Er Ke Za Zhi. 2020 Jul 2;58(7):591-595. doi: 10.3760/cma.j.cn112140-20200312-00221. Zhonghua Er Ke Za Zhi. 2020. PMID: 32605345 Review. Chinese.
-
Variability in clinical and neuropsychological features of individuals with MAP2K1 mutations.Am J Med Genet A. 2017 Feb;173(2):452-459. doi: 10.1002/ajmg.a.38044. Epub 2016 Nov 14. Am J Med Genet A. 2017. PMID: 27862862 Review.
Cited by
-
Genomic profiling informs diagnoses and treatment in vascular anomalies.Nat Med. 2023 Jun;29(6):1530-1539. doi: 10.1038/s41591-023-02364-x. Epub 2023 Jun 1. Nat Med. 2023. PMID: 37264205 Free PMC article.
-
Somatic activating BRAF variants cause isolated lymphatic malformations.HGG Adv. 2022 Mar 15;3(2):100101. doi: 10.1016/j.xhgg.2022.100101. eCollection 2022 Apr 14. HGG Adv. 2022. PMID: 35373151 Free PMC article.
-
Updates in Genetic Testing for Head and Neck Vascular Anomalies.Oral Maxillofac Surg Clin North Am. 2024 Feb;36(1):1-17. doi: 10.1016/j.coms.2023.09.001. Epub 2023 Oct 20. Oral Maxillofac Surg Clin North Am. 2024. PMID: 37867039 Free PMC article. Review.
-
Arteriovenous malformation Map2k1 mutation affects vasculogenesis.Sci Rep. 2023 Jul 8;13(1):11074. doi: 10.1038/s41598-023-35301-6. Sci Rep. 2023. PMID: 37422456 Free PMC article.
-
Combining Bioinformatics Techniques to Study the Key Immune-Related Genes in Abdominal Aortic Aneurysm.Front Genet. 2020 Dec 10;11:579215. doi: 10.3389/fgene.2020.579215. eCollection 2020. Front Genet. 2020. PMID: 33362847 Free PMC article.
References
-
- Wu JK, et al. Auricular arteriovenous malformation: Evaluation, management, and outcome. Plast. Reconstr. Surg. 2005;115:985–995. doi: 10.1097/01.PRS.0000154207.87313.DE. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

