Ventilator-associated pneumonia in adults: a narrative review
- PMID: 32157357
- PMCID: PMC7095206
- DOI: 10.1007/s00134-020-05980-0
Ventilator-associated pneumonia in adults: a narrative review
Abstract
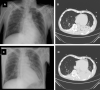
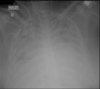
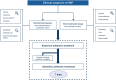
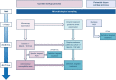
Ventilator-associated pneumonia (VAP) is one of the most frequent ICU-acquired infections. Reported incidences vary widely from 5 to 40% depending on the setting and diagnostic criteria. VAP is associated with prolonged duration of mechanical ventilation and ICU stay. The estimated attributable mortality of VAP is around 10%, with higher mortality rates in surgical ICU patients and in patients with mid-range severity scores at admission. Microbiological confirmation of infection is strongly encouraged. Which sampling method to use is still a matter of controversy. Emerging microbiological tools will likely modify our routine approach to diagnosing and treating VAP in the next future. Prevention of VAP is based on minimizing the exposure to mechanical ventilation and encouraging early liberation. Bundles that combine multiple prevention strategies may improve outcomes, but large randomized trials are needed to confirm this. Treatment should be limited to 7 days in the vast majority of the cases. Patients should be reassessed daily to confirm ongoing suspicion of disease, antibiotics should be narrowed as soon as antibiotic susceptibility results are available, and clinicians should consider stopping antibiotics if cultures are negative.
Keywords: Antibiotics; Bronchoalveolar lavage; Bronchoscopy; Endotracheal aspirate; Incidence; Mechanical ventilation; Mortality; Multiple-drug resistance; Prevention; Treatment; Ventilator-associated pneumonia; epidemiology.
Conflict of interest statement
LP received consultancy fees from Air Liquide MS, Faron, and MSD. CEL received fees from Bayer Healthcare in relationship with the current work (advisory board on inhaled amikacin), and fees from Merck, ThermoFischer Brahms, Biomérieux, Carmat, and Faron outside the current work. MK has received royalties from UpToDate Inc. for articles on ventilator-associated pneumonia.
Figures
References
-
- Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, Keohane C, Denham CR, Bates DW. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173:2039–2046. - PubMed
-
- American Thoracic Society, Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. - PubMed
-
- Reignier J, Mercier E, Le Gouge A, Boulain T, Desachy A, Bellec F, Clavel M, Frat JP, Plantefeve G, Quenot JP, Lascarrou JB, Clinical Research in Intensive C, Sepsis G (2013) Effect of not monitoring residual gastric volume on risk of ventilator-associated pneumonia in adults receiving mechanical ventilation and early enteral feeding: a randomized controlled trial. JAMA 309: 249–256 - PubMed
-
- Seguin P, Laviolle B, Dahyot-Fizelier C, Dumont R, Veber B, Gergaud S, Asehnoune K, Mimoz O, Donnio PY, Bellissant E, Malledant Y, Study of Povidone Iodine to Reduce Pulmonary Infection in Head T, Cerebral Hemorrhage Patients ICUSG, AtlanRea G (2014) Effect of oropharyngeal povidone-iodine preventive oral care on ventilator-associated pneumonia in severely brain-injured or cerebral hemorrhage patients: a multicenter, randomized controlled trial. Crit Care Med 42: 1–8 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

