Cancer testis antigen Cyclin A1 harbors several HLA-A*02:01-restricted T cell epitopes, which are presented and recognized in vivo
- PMID: 32157447
- PMCID: PMC8222032
- DOI: 10.1007/s00262-020-02519-6
Cancer testis antigen Cyclin A1 harbors several HLA-A*02:01-restricted T cell epitopes, which are presented and recognized in vivo
Erratum in
-
Correction to: Cancer testis antigen Cyclin A1 harbors several HLA‑A*02:01‑restricted T cell epitopes, which are presented and recognized in vivo.Cancer Immunol Immunother. 2021 Oct;70(10):3055. doi: 10.1007/s00262-021-02977-6. Cancer Immunol Immunother. 2021. PMID: 34170381 Free PMC article. No abstract available.
Abstract
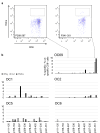
Cyclin A1 is a promising antigen for T cell therapy being selectively expressed in high-grade ovarian cancer (OC) and acute myeloid leukemia (AML) stem cells. For adoptive T cell therapy, a single epitope has to be selected, with high affinity to MHC class I and adequate processing and presentation by malignant cells to trigger full activation of specific T cells. In silico prediction with three algorithms indicated 13 peptides of Cyclin A1 9 to 11 amino acids of length to have high affinity to HLA-A*02:01. Ten of them proved to be affine in an HLA stabilization assay using TAP-deficient T2 cells. Their immunogenicity was assessed by repetitive stimulation of CD8+ T cells from two healthy donors with single-peptide-pulsed dendritic cells or monocytes. Intracellular cytokine staining quantified the enrichment of peptide-specific functional T cells. Seven peptides were immunogenic, three of them against both donors. Specific cell lines were cloned and used in killing assays to demonstrate recognition of endogenous Cyclin A1 in the HLA-A*02:01-positive AML cell line THP-1. Immunopeptidome analysis based on direct isolation of HLA-presented peptides by mass spectrometry of primary AML and OC samples identified four naturally presented epitopes of Cyclin A1. The immunopeptidome of HeLa cells transfected with Cyclin A1 and HLA-A*02:01 revealed six Cyclin A1-derived HLA ligands. Epitope p410-420 showed high affinity to HLA-A*02:01 and immunogenicity in both donors. It proved to be naturally presented on primary AML blast and provoked spontaneous functional response of T cells from treatment naïve OC and, therefore, warrants further development for clinical application.
Keywords: Acute myeloid leukemia; CD8+ T cell; Cyclin A1; Epitope; HLA immunopeptidome; Ovarian carcinoma.
Conflict of interest statement
AL received honorarium from the companies BMS, MSD, Roche, Tesaro, Novartis, Astra Zeneca, Grünenthal. HS is employee of Immatics Biotechnologies GmbH. His work in conjunction with this article was solely performed as a member of the Universities’ Department of Immunology. SO holds a patent covering Cyclin A1-targeted T cell immunotherapy for cancer. The other authors declare no conflict of interest.
Figures

Similar articles
-
Cyclin-A1 represents a new immunogenic targetable antigen expressed in acute myeloid leukemia stem cells with characteristics of a cancer-testis antigen.Blood. 2012 Jun 7;119(23):5492-501. doi: 10.1182/blood-2011-07-365890. Epub 2012 Apr 23. Blood. 2012. PMID: 22529286 Free PMC article.
-
T cell responses to HLA-A*0201-restricted peptides derived from human alpha fetoprotein.J Immunol. 2001 Apr 15;166(8):5300-8. doi: 10.4049/jimmunol.166.8.5300. J Immunol. 2001. PMID: 11290817
-
Recognition of PSA-derived peptide antigens by T cells from prostate cancer patients without any prior stimulation.Cancer Immunol Immunother. 2003 Aug;52(8):497-505. doi: 10.1007/s00262-003-0377-8. Epub 2003 Jun 3. Cancer Immunol Immunother. 2003. PMID: 12783216 Free PMC article.
-
Contemplating immunopeptidomes to better predict them.Semin Immunol. 2023 Mar;66:101708. doi: 10.1016/j.smim.2022.101708. Epub 2023 Jan 6. Semin Immunol. 2023. PMID: 36621290 Review.
-
Predicting Antigen Presentation-What Could We Learn From a Million Peptides?Front Immunol. 2018 Jul 25;9:1716. doi: 10.3389/fimmu.2018.01716. eCollection 2018. Front Immunol. 2018. PMID: 30090105 Free PMC article. Review.
Cited by
-
Target Selection for T-Cell Therapy in Epithelial Ovarian Cancer: Systematic Prioritization of Self-Antigens.Int J Mol Sci. 2023 Jan 24;24(3):2292. doi: 10.3390/ijms24032292. Int J Mol Sci. 2023. PMID: 36768616 Free PMC article.
-
Immune Surveillance of Acute Myeloid Leukemia Is Mediated by HLA-Presented Antigens on Leukemia Progenitor Cells.Blood Cancer Discov. 2023 Nov 1;4(6):468-489. doi: 10.1158/2643-3230.BCD-23-0020. Blood Cancer Discov. 2023. PMID: 37847741 Free PMC article.
-
Solving an MHC allele-specific bias in the reported immunopeptidome.JCI Insight. 2020 Oct 2;5(19):e141264. doi: 10.1172/jci.insight.141264. JCI Insight. 2020. PMID: 32897882 Free PMC article.
-
Immunopeptidomic Analysis of BoLA-I and BoLA-DR Presented Peptides from Theileria parva Infected Cells.Vaccines (Basel). 2022 Nov 11;10(11):1907. doi: 10.3390/vaccines10111907. Vaccines (Basel). 2022. PMID: 36423003 Free PMC article.
-
PRAME and CTCFL-reactive TCRs for the treatment of ovarian cancer.Front Immunol. 2023 Mar 21;14:1121973. doi: 10.3389/fimmu.2023.1121973. eCollection 2023. Front Immunol. 2023. PMID: 37026005 Free PMC article.
References
-
- Chae YK, Arya A, Iams W, Cruz MR, Chandra S, Choi J, Giles F. Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer; lessons learned from clinical trials with melanoma and non-small cell lung cancer (NSCLC) J Immunother Cancer. 2018 doi: 10.1186/s40425-018-0349-3. - DOI - PMC - PubMed
-
- Park JH, Riviere I, Gonen M, Wang X, Senechal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E, Roshal M, Maslak P, Davila M, Brentjens RJ, Sadelain M. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449–459. doi: 10.1056/NEJMoa1709919. - DOI - PMC - PubMed
-
- Robbins PF, Kassim SH, Tran TL, Crystal JS, Morgan RA, Feldman SA, Yang JC, Dudley ME, Wunderlich JR, Sherry RM, Kammula US, Hughes MS, Restifo NP, Raffeld M, Lee CC, Li YF, El-Gamil M, Rosenberg SA. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res: Off J Am Assoc Cancer Res. 2015;21(5):1019–1027. doi: 10.1158/1078-0432.CCR-14-2708. - DOI - PMC - PubMed
-
- Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, Kammula US, Hughes MS, Restifo NP, Raffeld M, Lee CC, Levy CL, Li YF, El-Gamil M, Schwarz SL, Laurencot C, Rosenberg SA. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol: Off J Am Soc Clin Oncol. 2011;29(7):917–924. doi: 10.1200/JCO.2010.32.2537. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

