A Mother-to-Child Transmission Study in Nigeria: The Impact of Maternal HIV Infection and HAART on Plasma Immunoglobulins, Cytokine Profiles and Infant Outcome
- PMID: 32157604
- PMCID: PMC7462942
- DOI: 10.1007/s12250-020-00202-9
A Mother-to-Child Transmission Study in Nigeria: The Impact of Maternal HIV Infection and HAART on Plasma Immunoglobulins, Cytokine Profiles and Infant Outcome
Abstract
Prevention of mother-to-child transmission (PMTCT) of HIV with highly active antiretroviral therapy (HARRT) allows the HIV+ pregnant mothers to have vaginal delivery and breastfeed. Here we investigated the maternal plasma immunoglobulin, cytokine secretion and the outcome of the exposed infants among the HIV+ HAART treated pregnant women in Nigeria. In this study, different plasma immunoglobulins and cytokines were measured in the HIV+ HAART treated pregnant mothers. Pooled culture supernatants of B and T lymphocytes showed lower levels of IFN-γ, IL-10 and IL-4. There were lower IFN-γ and IL-10 secretions at 1st trimester; however, IL-10 continued to be lower throughout 2nd and 3rd trimesters. TNF-α secretion significantly decreased as pregnancy progressed to term. There were high plasma IgG and low IgM in the HIV+ HAART treated pregnant women. Plasma IgG was high during 1st and 3rd trimesters. After one year of follow up, all the exposed children were seronegative for HIV-1 and HIV-2. Vaginal delivery and breastfeeding among HIV+ HAART treated mothers have shown to be safe. The use of HAART by the infected mothers and the use of septrin and niverapin by the exposed infants prevented mother to-child transmission of HIV.
Keywords: Cytokine; Highly active antiretroviral therapy (HAART); Human immunodeficiency virus (HIV); Immunoglobulins; Lymphocyte stimulation; Mitogen; Prevention from mother-to-child transmission (PMTCT).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
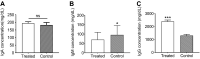
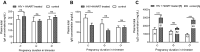
References
-
- Borges-Almeida E, Milanez HM, Vilela MM, Cunha FG, Abramczuk BM, Reis-Alves SC, Metze K, Lorand-Metze I. The impact of maternal HIV infection on cord blood lymphocyte subsets and cytokine profile in exposed non-infected newborns. BMC Infect Dis. 2011;11:38. doi: 10.1186/1471-2334-11-38. - DOI - PMC - PubMed
-
- Burns WR, Wang Y, Tang PC, Ranjbaran H, Iakimov A, Kim J, Cuffy M, Bai Y, Pober JS, Tellides G. Recruitment of CXCR3+ and CCR5+ T cells and production of interferon-gamma-inducible chemokines in rejecting human arteries. Am J Transpl. 2005;5:1226–1236. doi: 10.1111/j.1600-6143.2005.00892.x. - DOI - PubMed
-
- Clerici M, Seminari E, Suter F, Castelli F, Pan A, Biasin M, Colombo F, Trabattoni D, Maggiolo F, Carosi G, Maserati R. Different immunologic profiles characterize HIV infection in highly active antiretroviral therapy-treated and antiretroviral-naive subjects with undetectable viremia. AIDS. 2000;14:109–116. doi: 10.1097/00002030-200001280-00005. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

